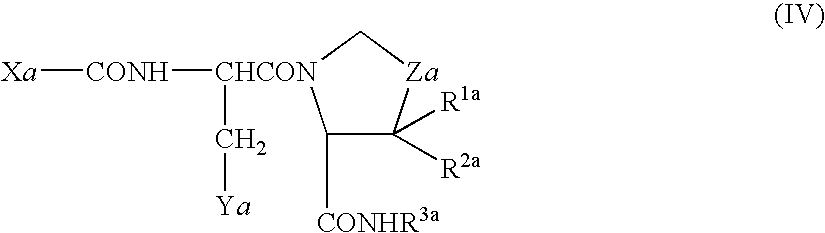
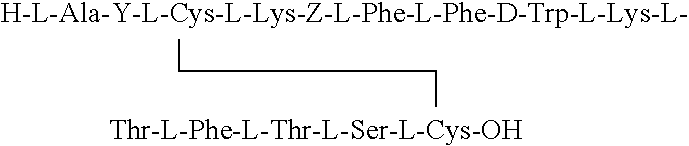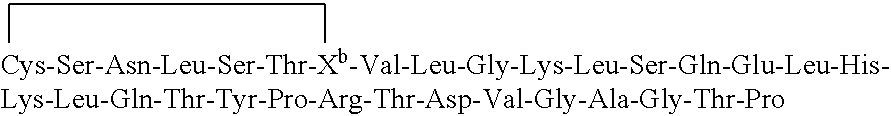Composition and device structure for iontophoresis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experimental example 1
A Preparation Example when Aminoalkylmethacrylate Copolymer E is Used as a pH Adjusting Agent
[0133] Experimental Example 1 shows a preparation example when aminoalkylmethacrylate copolymer E is used as a pH adjusting agent. The pH of a preparation in this Experimental Example was adjusted to around 4.5-6.5. In this step, hydrochloric acid was charged to add chloride ion as a reactant with an active electrode.
example 1
[0134]
5 Component Content (%(w / w)) Fentanyl citrate 1.00 Agar 1.00 Aminoalkylmethacrylate copolymer E 5.60 Hydrochloric acid 0.55 Methyl para-oxybenzoate 0.20 Distilled water for injection Appropriate amount Total 100.00
[0135] Fentanyl citrate, aminoalkylmethacrylate copolymer E and hydrochloric acid were added to distilled water and stirred until these were dissolved. Further, agar and methyl para-oxybenzoate were added with stirring followed by heating at about 90.degree. C. to dissolve. After cooled near to about 60.degree. C., about 0.8 g of this solution was charged into an electrode cup made of polyethylene terephthalate (thereinafter, abbreviated as PET). The pH of the prepared formulation was 4.9.
experimental example 2
Influence of Aminoalkylmethacrylate Copolymer E Affecting Aniontophoresis-Releasing Test of Fentanyl Citrate
[0140] Next, using the above described formulations (Example 1 and Comparative Example 2), a releasing test by iontophoresis was performed. Using a sideways type cell in the releasing test, 3% (w / w) agarose gel (3 mm) was used as a release control layer. The experiment was conducted at a room temperature, and after electrically energizing with current for 1 hr, the drug amount transferred into a receptor layer was measured by high-performance liquid chromatography. The electrically energizing with current was performed at 0.2 mA / cm.sup.2 constant-current for 1 hr of the total energizing time using direct-current energizing for pulse depolarization (50 kHz frequency, 50% duty) by a short-circuit switch. FIG. 2 is a graph showing the results of the releasing test of fentanyl citrate by iontophoresis. As clarified from FIG. 2, the releasing amount of fentanyl by ionyophoresis is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com