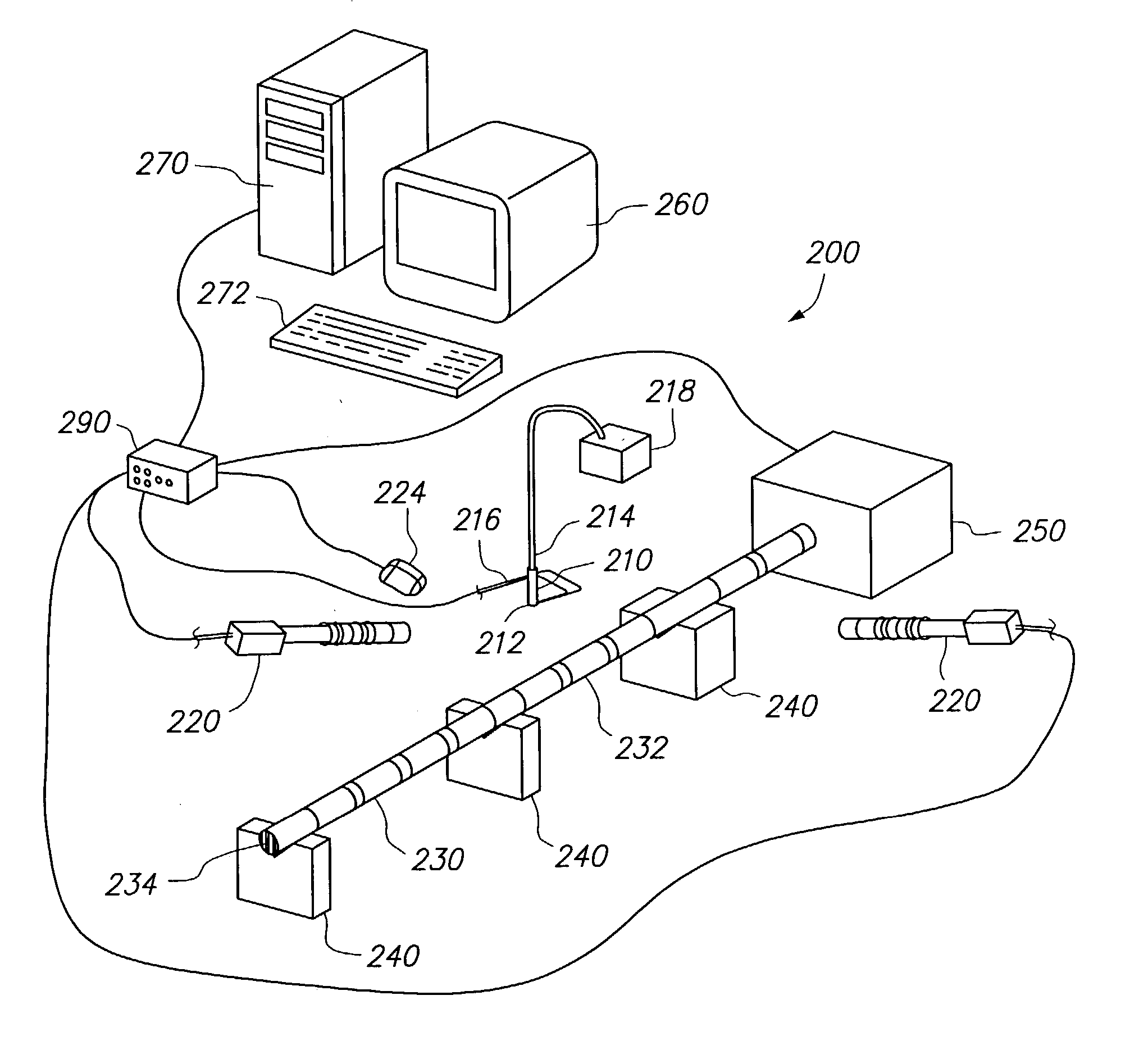
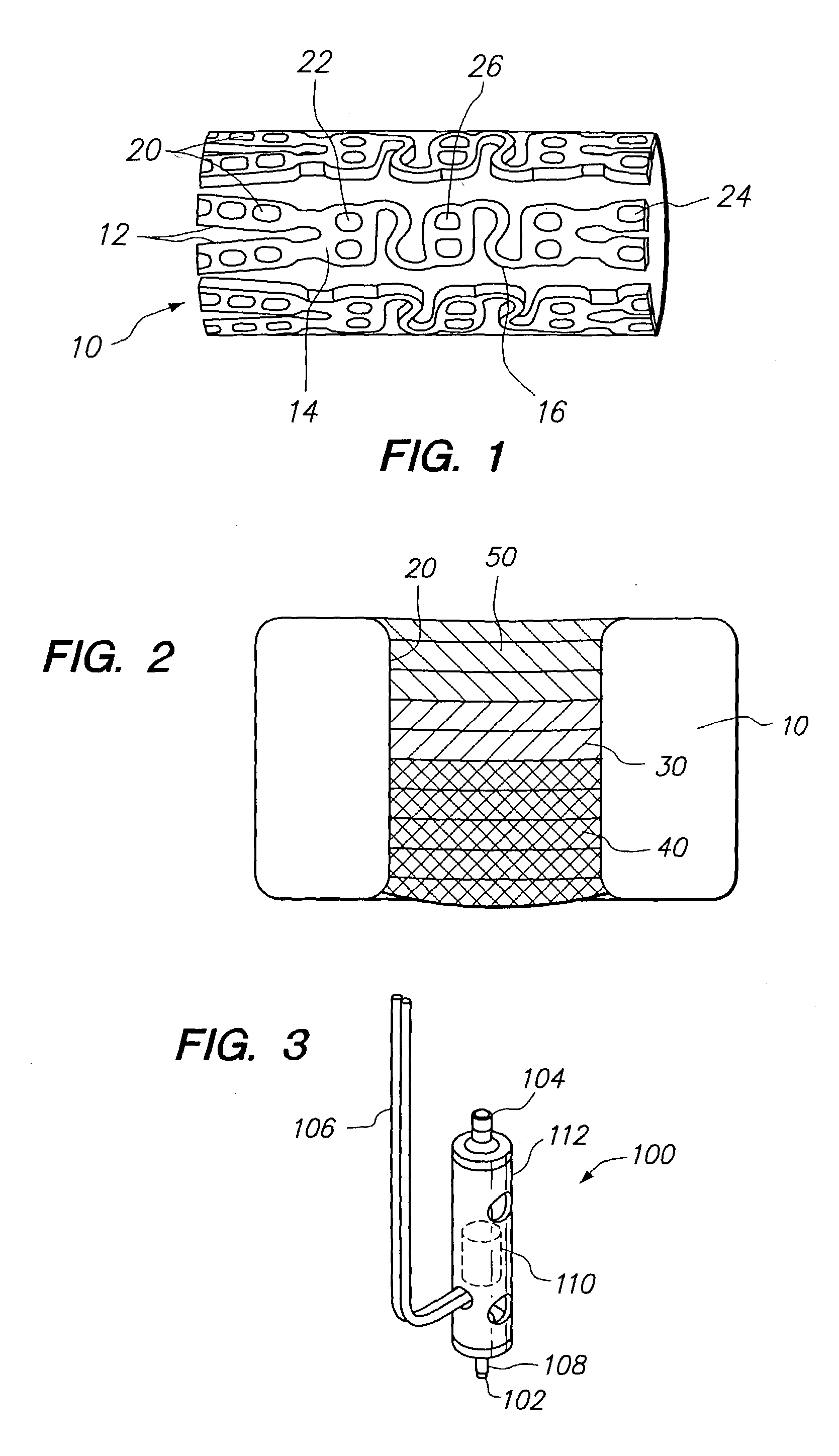
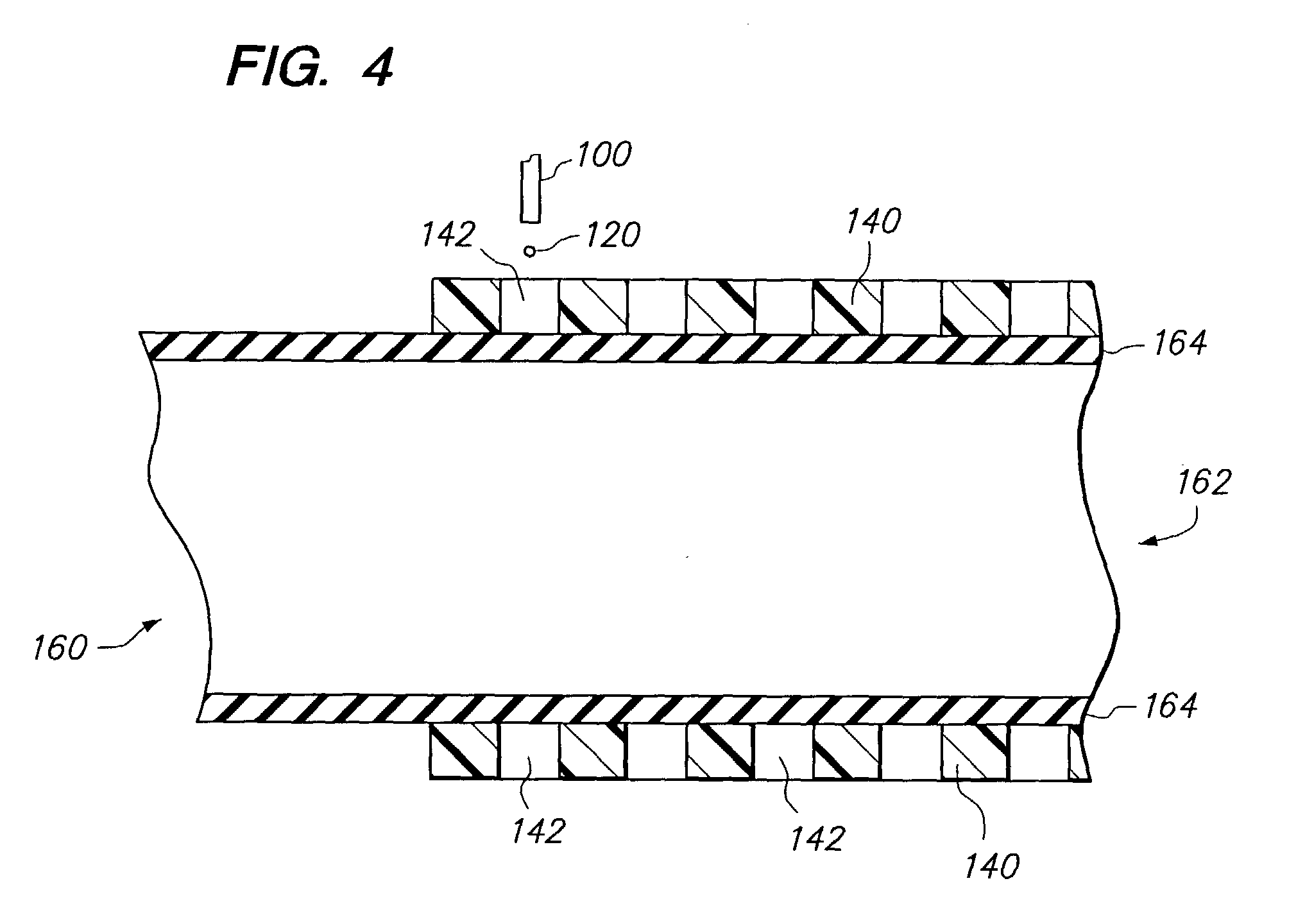
Method and apparatus for loading a beneficial agent into an expandable medical device
a medical device and beneficial agent technology, applied in the direction of dilators, prostheses, blood vessels, etc., can solve the problems of increasing trauma and risk to patients, surface coating, and major complications of stenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0088] In the example below, the following abbreviations have the following meanings. If an abbreviation is not defined, it has its generally accepted meaning.
1 TABLE I Solutions Drug Polymer Solvent A None 4% PLGA DMSO 50 / 50 IV = 0.82 DA 0.64% 8% PLGA DMSO paclitaxel 50 / 50 IV = 0.59 DA 0.64% 8% PLGA DMSO paclitaxel 50 / 50 IV = 0.60 DD 0.14% 8% PLGA DMSO paclitaxel 50 / 50 IV = 0.59 L None 8% PLGA DMSO 50 / 50 IV = 0.59 DMSO = Dimethyl Sulfoxide IV = Inherent Viscosity PLGA = poly(lactide-co-glycolide)
[0089]
2TABLE II Layer No., this Layer No. Solution Solution 1 A 1 2 A 2 3 A 3 4 A 4 5 A 5 6 A 6 7 A 7 8 A 8 9 A 9 10 DA 1 11 DA 2 12 DD 1 13 L 1
[0090] A plurality of medical devices, preferably 11 medical devices per mandrel are placed onto a series of mandrels. Each mandrel is bar coded with a unique indicia which identifies at least the type of medical device, the layers of beneficial agents to be loaded into the opening of the medical devices, and a specific identity for each mandrel. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Shape | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com