Embolic filter frame having looped support strut elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
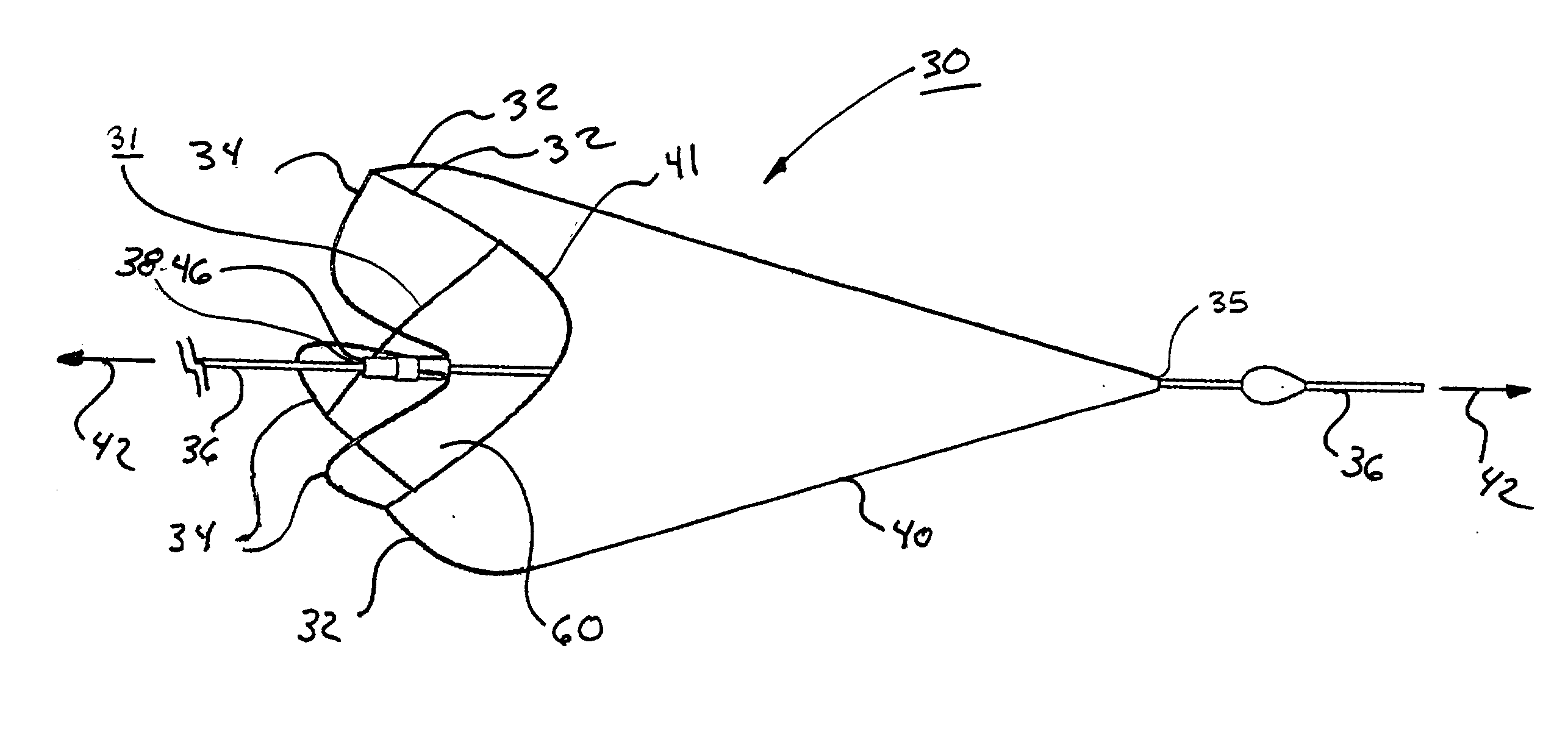
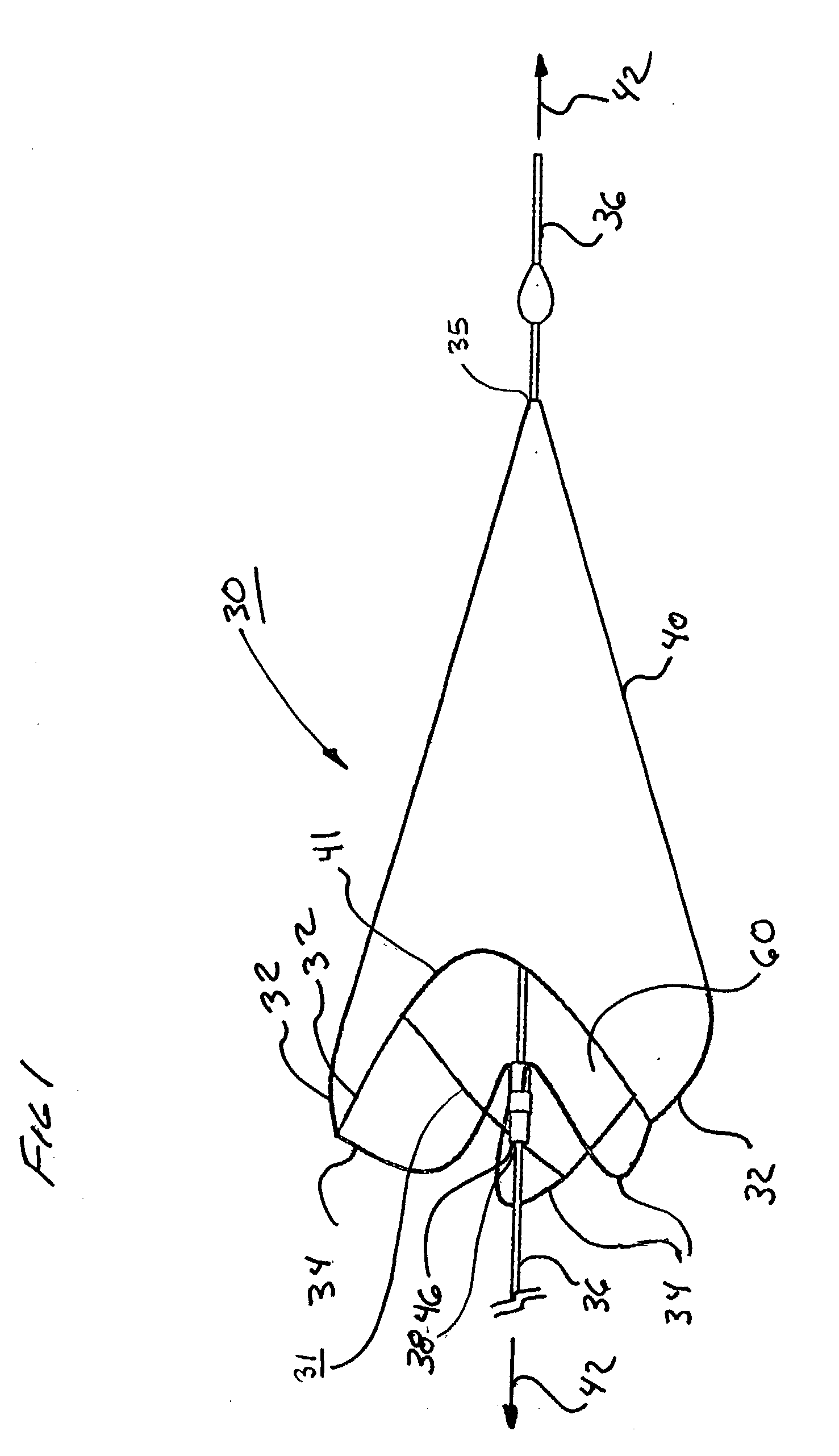
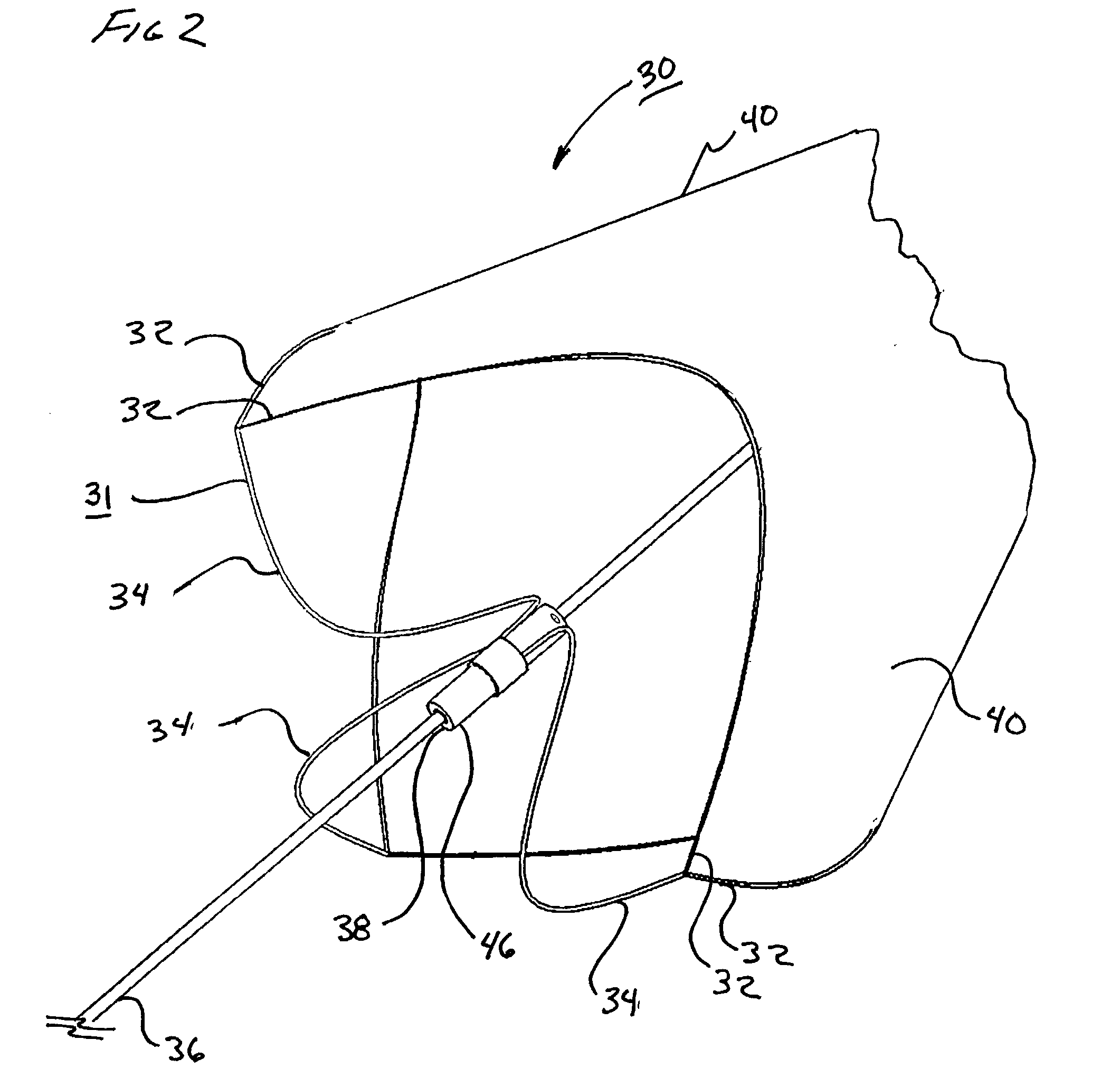
[0095] As shown in FIG. 15, a 0.9 mm nitinol tube 104, with a wall thickness of approximately 0.09 mm (obtained from SMA Inc, San Jose, Calif.) was laser cut by Laserage Technologies Inc, Waukegan, Ill., to form a frame configuration of a single, undulating, integral, 6 apex ring. The frame included radiopaque marker housings 106 at each distal apex and tether or strut elements 34 extending from each proximal apex 108 and converging at the opposite end in a "collar" 46 of uncut parent material. This frame was then lightly grit blasted at 30 psi with 20-micron silicon carbide media in a grit blasting machine (Model MB1000 available from Comco Inc, Burbank, Calif.). The frame was then gently slid up a tapered mandrel until it achieved a functional size of approximately 6mm.
[0096] The frame and mandrel were then subjected to an initial thermal treatment to set the geometry in an initial, tapered (conical) configuration in an air convection oven (Carbolite Corporation, Sheffield, Englan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com