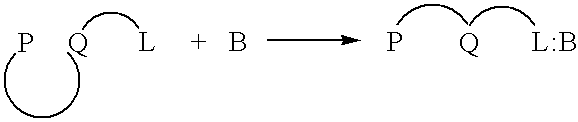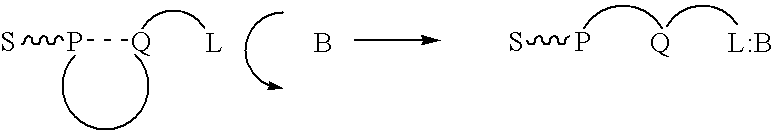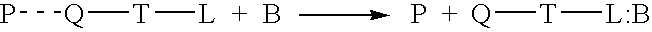Fluorescent polymer-QTL approach to biosensing
a polymer-qtl and biosensor technology, applied in the field of fluorescent biosensors, can solve the problems of important limitations and differentiations to fret relative to the polymer-qtl approach, cumbersome and time-consuming, and achieve the effect of enhancing the detection of target molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Anchored Polymer-tethered QTL Approach
[0107] In an aqueous solution, anionic polymer 1 illustrated in FIG. 11 is strongly quenched by cationic electron acceptors such as methyl viologen (Formula 3 illustrated in FIG. 11) or the viologen-biotin conjugate, Formula 4, shown in FIG. 11. (Chen, L.; McBranch. D. W.; Wang. H.-L.; Helgeson. R.; Wudl. F.; Whitten, D. G. Proc. Natl. Acad. Sci. 1999, 96, 12287-12292: Whitten. D.; Chen. L.; Jones. R.; Bergstedt, T.; Heeger. P.; and McBranch. D. in "Sensors and Optical Switches", Molecular and Supramolecular Photochemistry Vol. 7, Eds. K. S. Schanze and V. Ramamurthy, Marcel Dekker, Inc. Pub. In press).
[0108] As shown previously, the "unquenching" observed when the protein avidin is added to quenched solutions of polymer 1 complexed with QTL 4 provides a quantitative assay for the sensitive detection of a protein. Similarly, the cationic cyanine polymer 2 exhibits superquenching with the anionic anthraquinone disulfonate 5 and the corresponding ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight cutoff | aaaaa | aaaaa |
| Q-average size | aaaaa | aaaaa |
| fluorescent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com