Pyridoxamine for the treatment of diabetic kidney disease
a technology of pyridoxamine and kidney disease, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of poor renal function, poor renal function, and inability to effectively filter blood in the glomerulus of patients, so as to and limit the progression of renal disease and/or diabetic complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
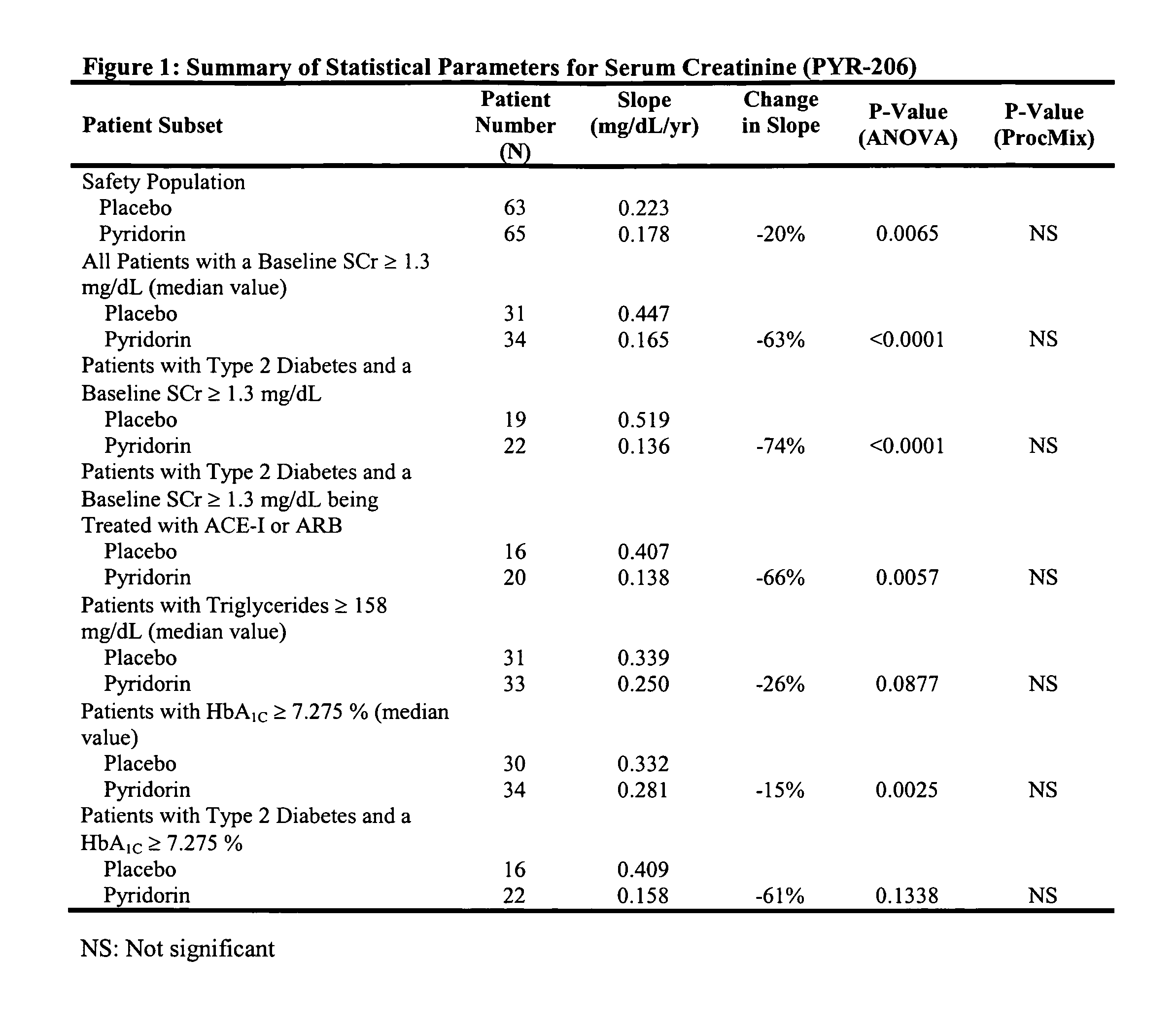
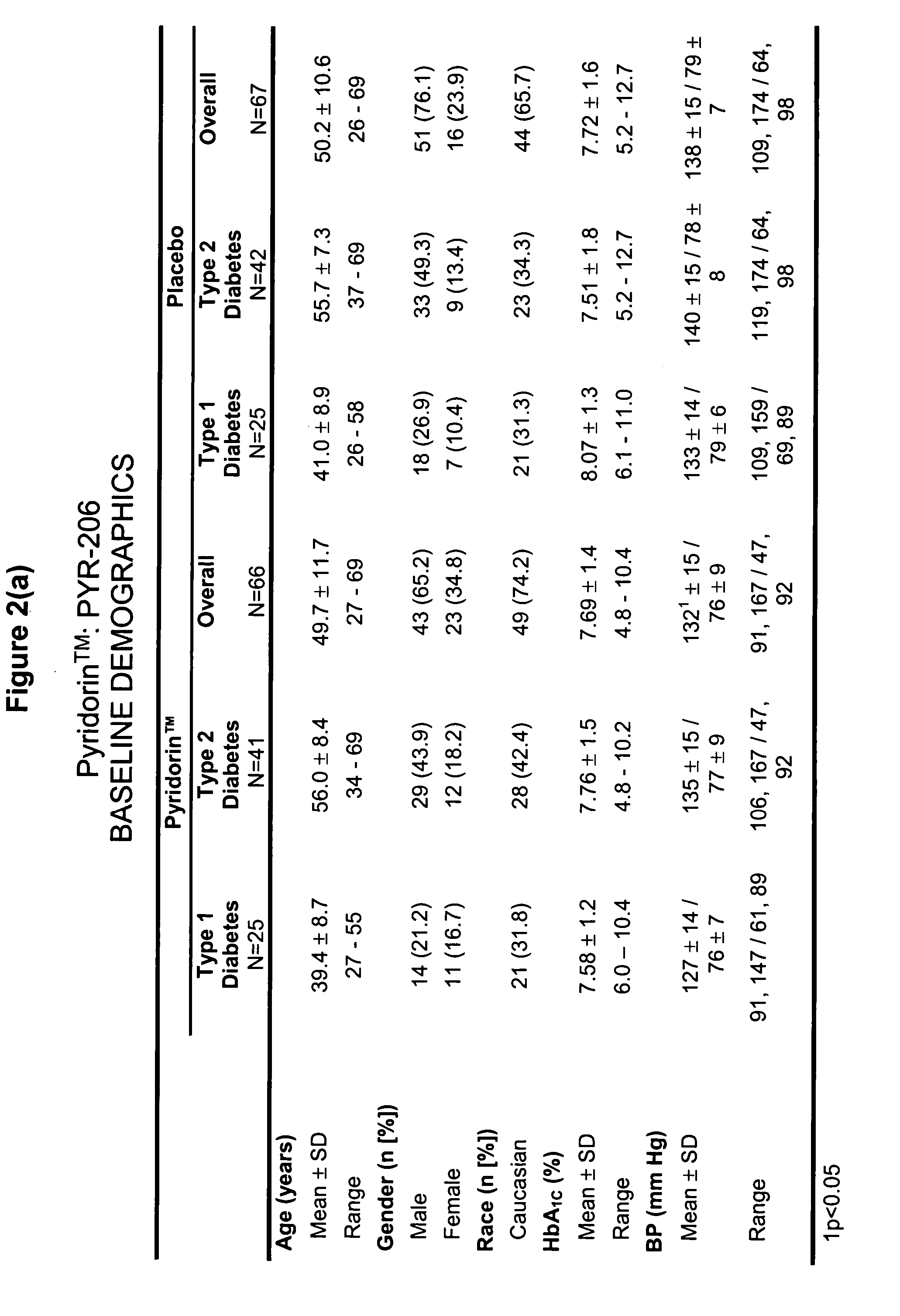
[0051] A randomized, double-blind, placebo-controlled, multicenter trial which examined the safety profile of pyridoxamine dihydrochloride (PYR) in patients with type 1 and type 2 diabetes mellitus (“DM”) and overt nephropathy was conducted (“206 study”). 128 patients (48 type 1, 80 type 2) at 32 sites were randomized to receive either PYR 50 mg twice a day (b.i.d) or placebo for six months. 58 patients in each group completed the study. Groups were well matched at baseline for age, race, gender, blood pressure, hemoglobin A1C (HbA1C), and angiotensin converting enzyme inhibitor (ACEI) / angiotensin receptor blocker (ARB) use.
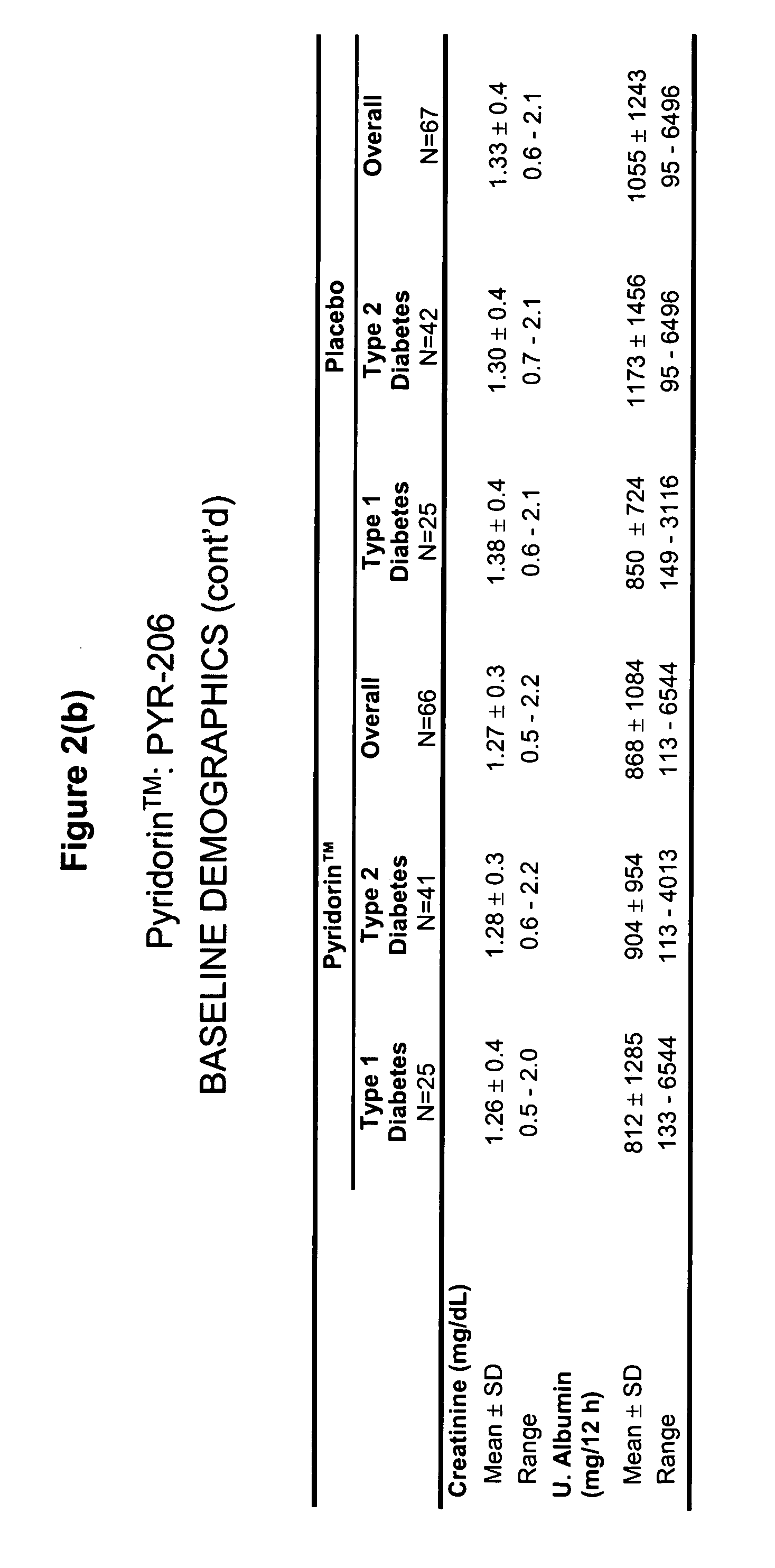
[0052] Baseline characteristics of the patients included serum creatinine=1.27 mg / dL and urinary albumin excretion=868 mg / 12 h in treatment, versus 1.33 mg / dL and 1055 mg / 12 h in placebo groups (differences not significant, NS).
[0053] No significant differences in treatment-related adverse events (26% PYR, 33% placebo), study discontinuation due to AE's (6% PYR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com