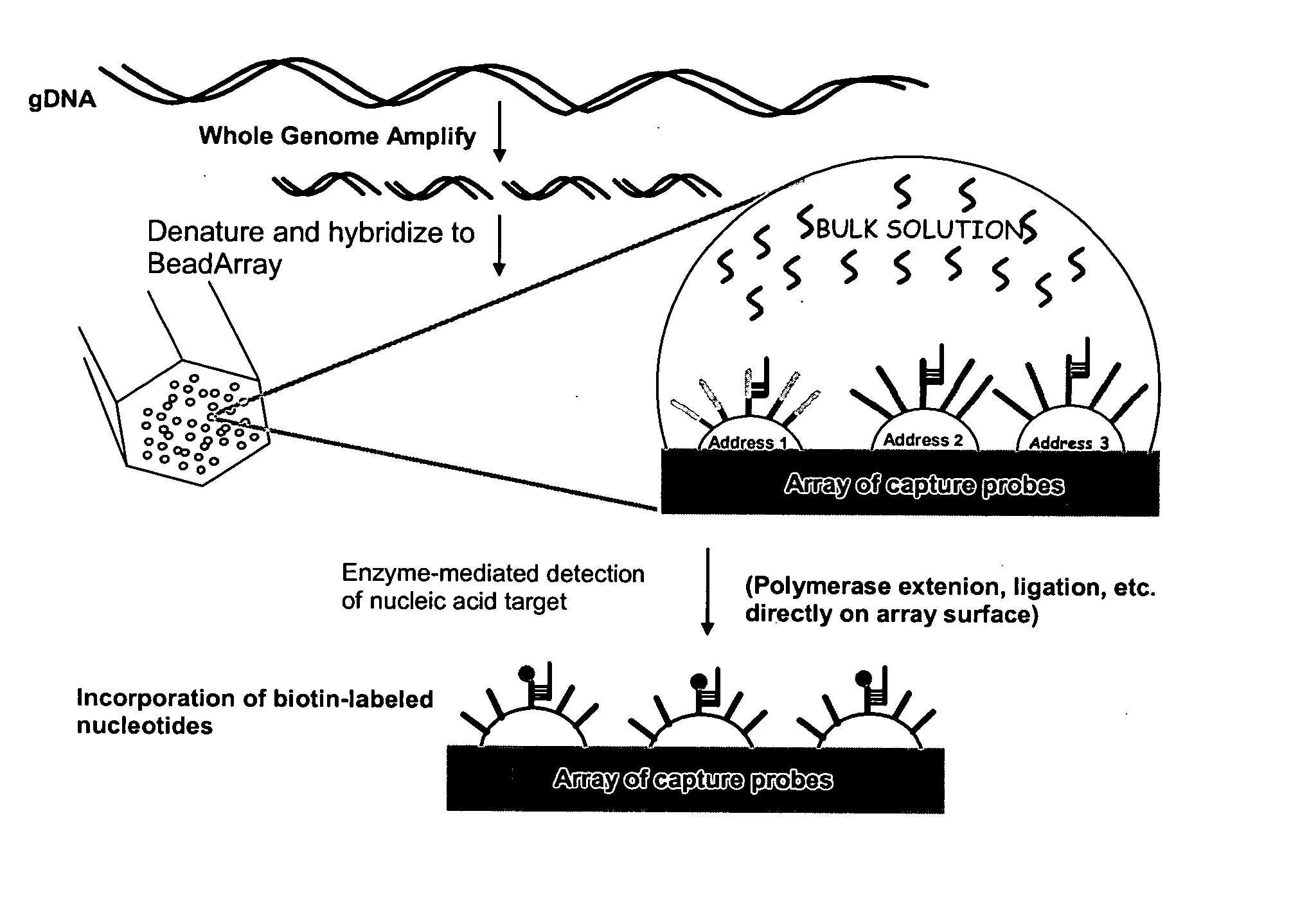
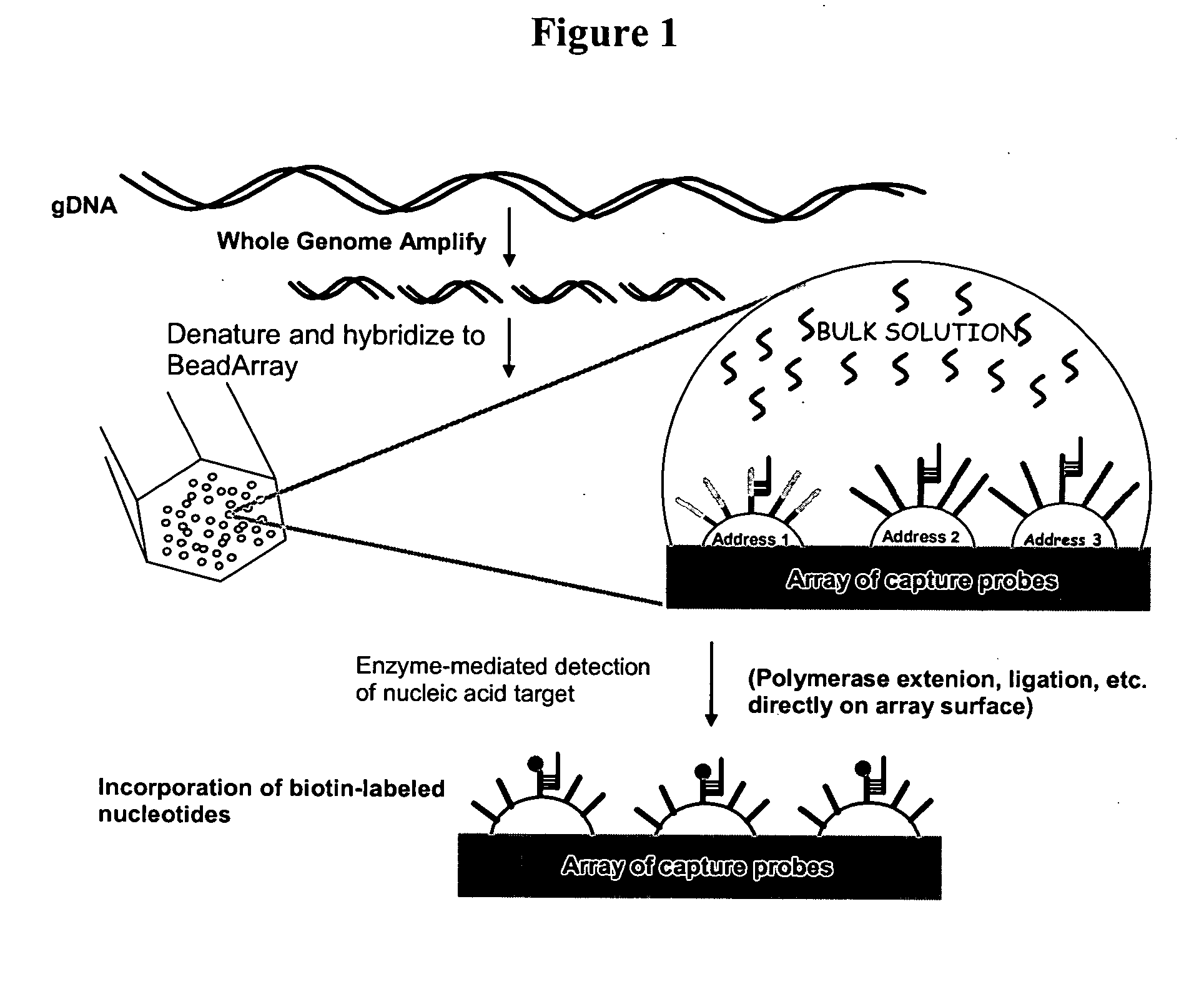
Methods and compositions for whole genome amplification and genotyping
a whole genome and genotyping technology, applied in the field of gene analysis, can solve the problems of inefficiency of current methods for genome-wide interrogation of snps and other markers, rendering the identification of useful diagnostic marker sets impractical, and affecting the accuracy of results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Whole Genome Amplification Using Random-Primed Amplification (RPA)
[0325] This example demonstrates production of an amplified representative population of genome fragments from a yeast genome.
[0326] Yeast genomic DNA, from S. Cerevisiae strain S228C, was prepared using a Qiagen Genomic DNA extraction kit and 10 ng of the genomic DNA was amplified with Klenow polymerase.
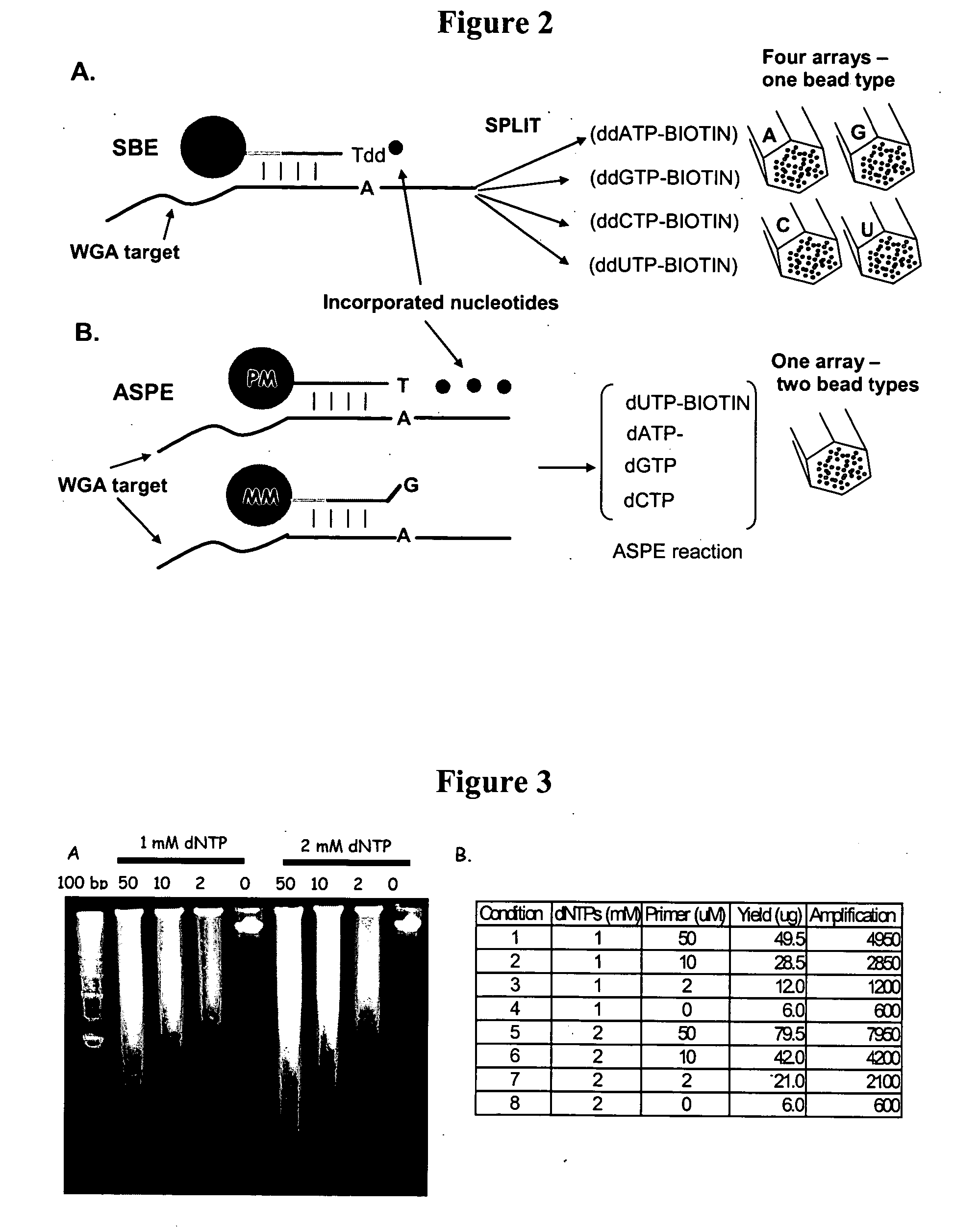
[0327] Several parameters were evaluated to determine their effect on the yield of the Klenow (exo−) random-primed amplification reaction. Amplification reactions were carried out under similar conditions with the exception that one parameter was systematically modified. FIG. 3 shows results comparing amplification reactions carried out at different concentrations of deoxynucleotide triphosphates.
[0328] Following each reaction, the amplified DNA was purified on Montage ultrafiltration plates (Millipore), loaded onto an agarose gel and the DNA quantitated by UV260 reading as shown in FIG. 3A. The amplification yiel...
example ii
Detection of Yeast Loci for a Yeast Whole Genome Sample Hybridized to BeadArrays™
[0330] This example demonstrates reproducible detection of yeast loci for a yeast whole genome sample hybridized to a BeadArrays™ and probed with allele-specific primer extension (ASPE).
[0331] Six hundred nanograms of random primer amplified (RPA) yeast gDNA was hybridized to a locus-specific BeadArray™ (Illumina). The BeadArray™ was composed of 96 oligonucleotide probe pairs (PM and MM, 50 bases in length) interrogating different gene-based loci distributed throughout the S. cerevisiae genome. The amplified yeast genomic DNA was hybridized to the BeadArray™ under the following conditions: Overnight hybridization at 48° C. in standard 1× hybridization buffer (1 M NaCl, 100 mM potassium-phosphate buffer (pH 7.5), 0.1% Tween 20, 20% formamide). After hybridization, arrays were washed in 1× hybridization buffer at 48° C. for 5 min. followed by a wash in 0.1× hybridization buffer at room temperature for mi...
example iii
Whole Genome Genotyping (WGG) of Human gDNA Directly Hybridized to BeadArrays™
[0336] This example demonstrates hybridization of a representative population of genome fragments to an array and direct detection of several typable loci of the hybridized genome fragments. This example further demonstrates detection of typable loci on an array using either of two different primer extension assays.
[0337] SBE-Based Detection
[0338] Human placental genomic DNA samples were obtained from Coriell Inst. Camden, N.J. The human placental gDNA sample (150 ug) was hybridized to a BeadArray™ (Illumina) having 4 separate bundles each containing the same set of 24 different non-polymorphic probes (50-mers). The BeadArray™ consisted of 96 probes to human non-polymorphic loci randomly distributed throughout the human genome. The probes were 50 bases long with ˜50% GC content and designed to resequence adjacent A (16 probes), C (16 probes), G (16 probes), or T (16 probes) bases. DNA samples (150 ug hum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com