Recombinant influenza virus vector carrying foreign genes in NA segment and preparation method and application thereof
An influenza virus and foreign gene technology, which is applied in the field of recombinant influenza virus as a viral vector and its preparation, can solve the problems of instability, high cost, and the inability of passage of recombinant influenza virus to achieve the effect of increasing the length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
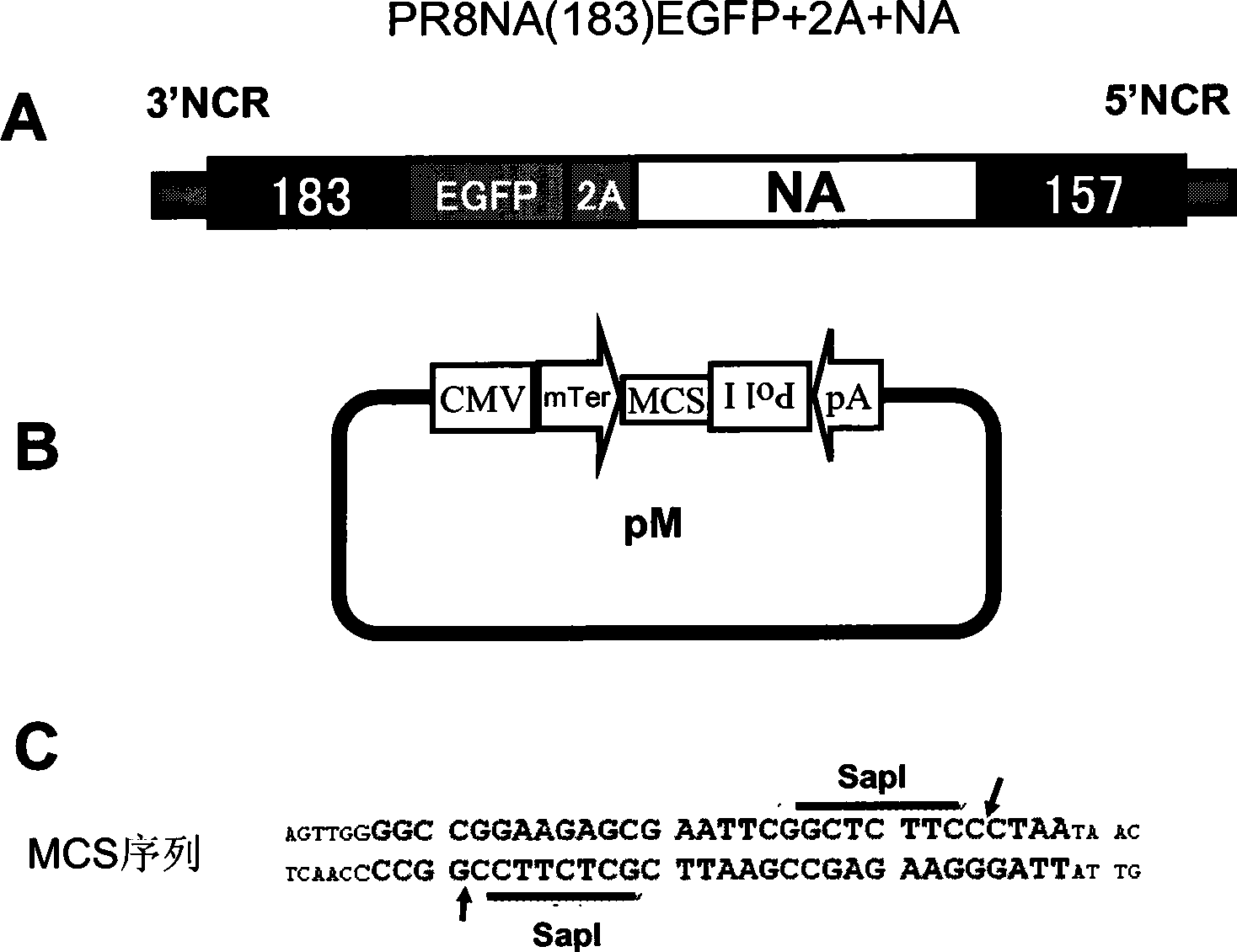
[0040] Example 1: Schematic diagram of the structure of an embedded NA fragment containing a foreign gene and a schematic diagram of the structure of a CMV / PolI bidirectional expression system.
[0041] figure 1 A, the composition of the whole recombinant NA segment from 3' to 5' is: the packaging signal of the NA gene segment (the 3' non-coding region of PR8NA and the first 183 nt of the coding region), the foreign protein expression frame (here preferably EGFP Reporter gene), 2A short peptide coding region, NA gene coding frame (ATG to stop codon, which includes 157nt packaging signal of coding region) and 5' non-coding region.
[0042] Schematic diagram of the structure of the bidirectional promoter CMV / Pol I ( figure 1 B), the specific structure is similar to that mentioned in the literature (Hoffmann, E., Neumann, G., Kawaoka, Y., Hobom, G., and Webster, R.G. (2000). A DNA transfection system for generation of influenza A virus from eight plasmamids.Proc Natl Acad Sci...
Embodiment 2
[0044] Example 2: Obtaining of DNA fragments fused with exogenous gene and 2A.
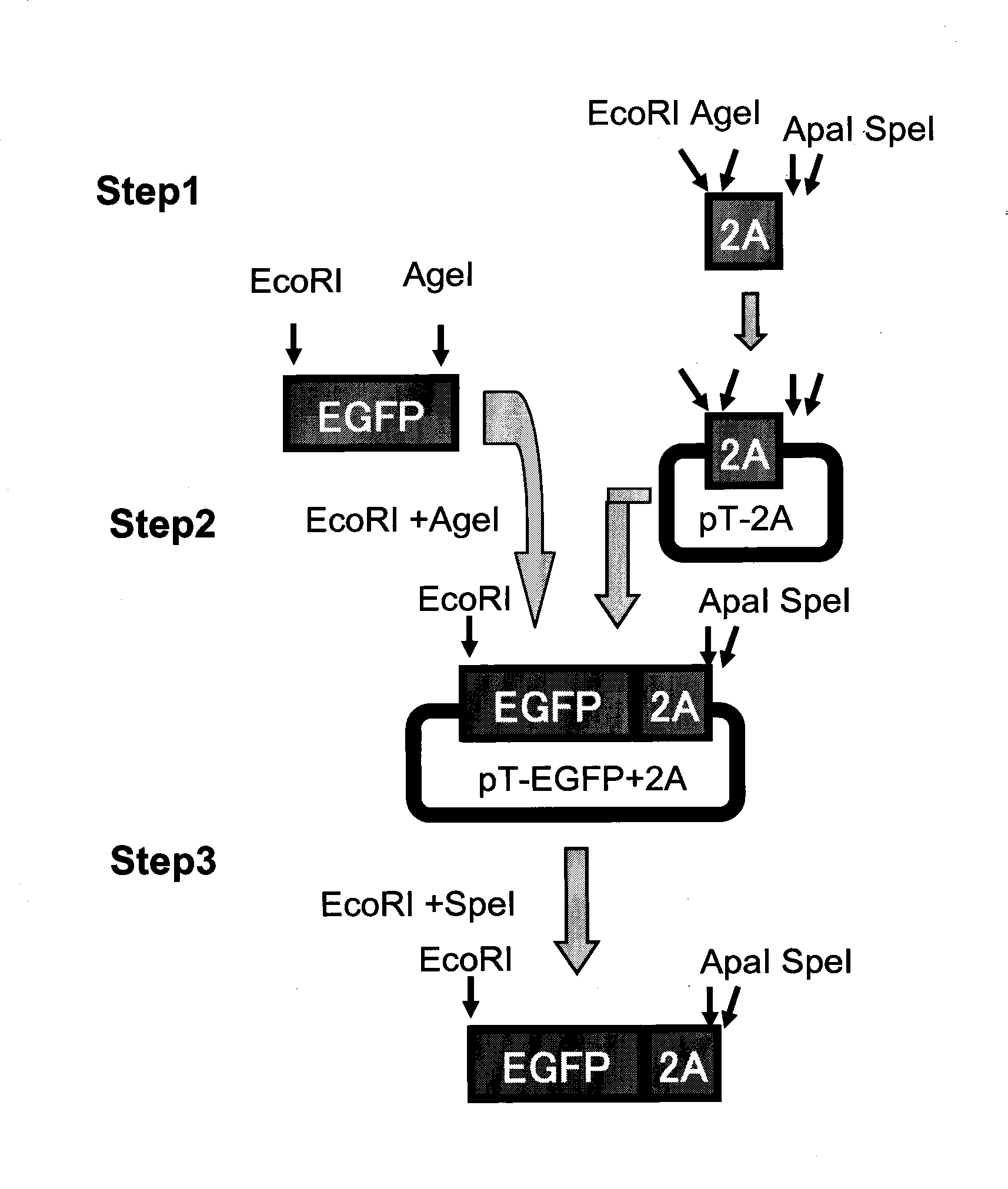
[0045] Synthesize two complementary oligonucleotide chains up and down, and form a double-stranded DNA fragment containing restriction site after annealing: GAATTC(EcoRI)agg ACCGGT(AgeI) tctggcgccaccaacttctccctgctgaa gcaggctggcgatgtggaggagaaccctGGGCCC (ApaI)atgACTAGT(SpeI) underlined part is 2A sequence. The double-stranded fragment was ligated and inserted into the pMD18T-simple vector (TaKaRa Company, Dalian), to obtain pT-2A ( figure 2 ), wherein EcoRI and AgeI sites were introduced at the 5' end, and ApaI and SpeI sites were introduced at the 3' end.
[0046] Using pCDNA-EGFP as a template, the EGFP reporter gene was amplified by PCR, the primers were GFP(1+20)EI and GFP(720-24)Age and the EcoRI site introduced at the 5' end and the AgeI site introduced at the 3' end , inserted into pT-2A to obtain pT-EGFP-2A ( figure 2 ). The EGFP+2A fusion fragment was excised by EcoRI and SpeI for ...
Embodiment 3
[0049] Example 3: Construction of pM-PR8NA(183)EGFP+2A+NA plasmid.
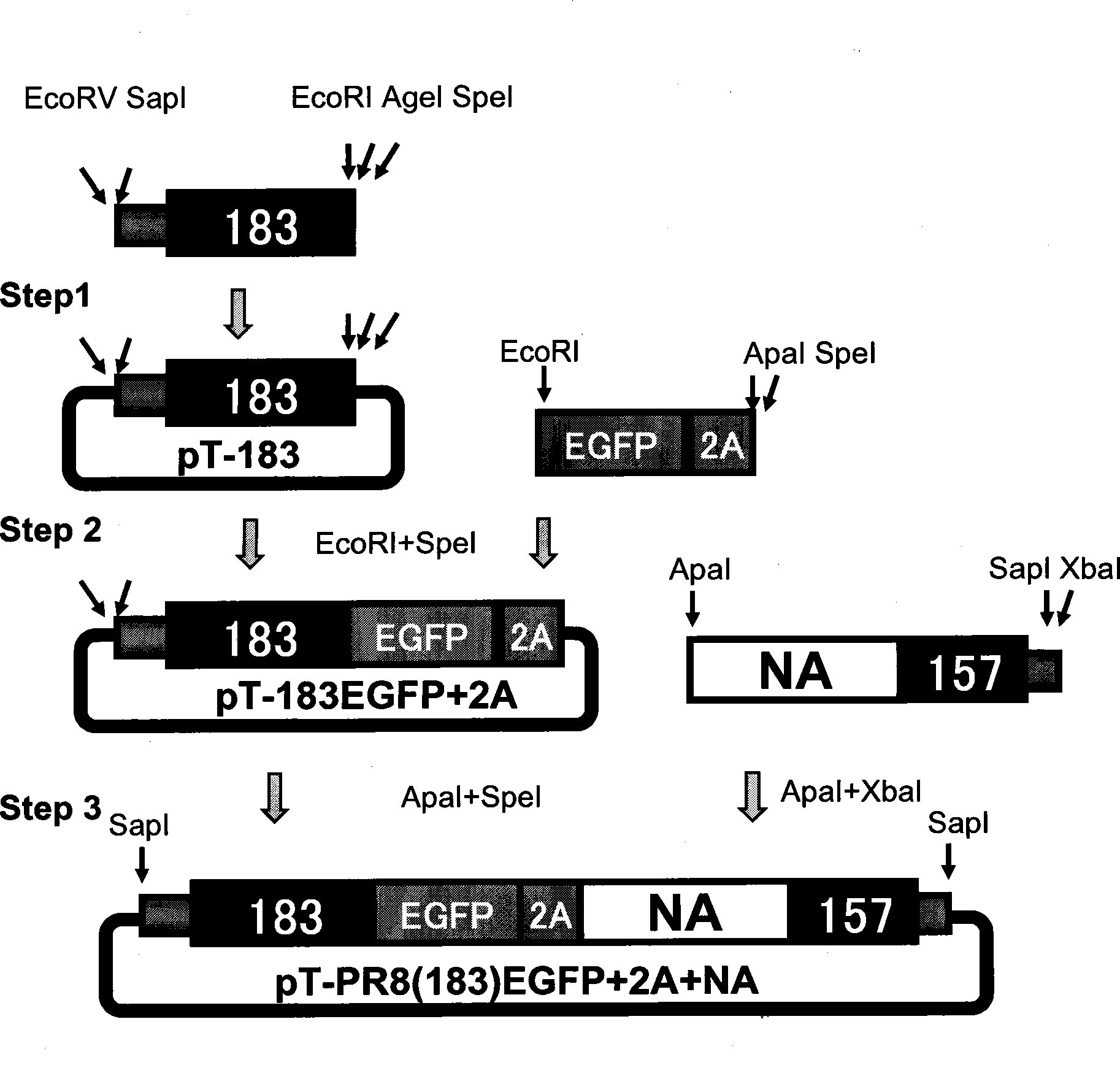
[0050]Using the plasmid pPolI-PR8NA containing the full-length PR8NA gene fragment as a template, using Sap-NA(1+20)EcoRV and PR8NA(183-24)EiAS as a primer pair, PCR technology was used to amplify the 3' end of the NA fragment of influenza virus Selectively package the signal part, including the 3' non-coding region and the first 183 nucleotides of the 3' coding region, and introduce EcoRV and SapI sites at one end of the non-coding region, and introduce EcoRI sites and AgeI at the 183 end site and SpeI site. Insert the pMD18T-simple vector to obtain pT-183 ( image 3 ).
[0051] pT-183 was digested with EcoRI and SpeI, and inserted into the EGFP+2A fusion fragment obtained in Example 2 to obtain pT-183EGFP+2A ( image 3 ).
[0052] Using the above-mentioned plasmid pPolI-PR8NA as a template, using PR8NA(1+26)ApaI and Sap-NA-1413R-XbaI as a primer pair, the first codon ATG to 5 of the influenza virus NA g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com