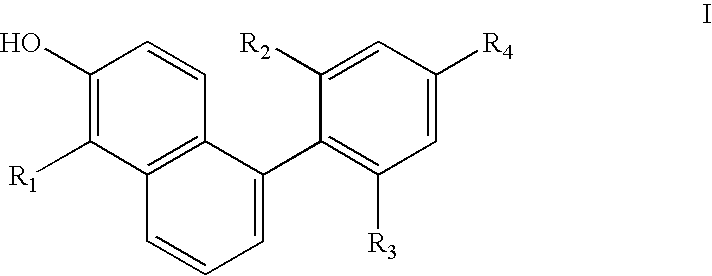
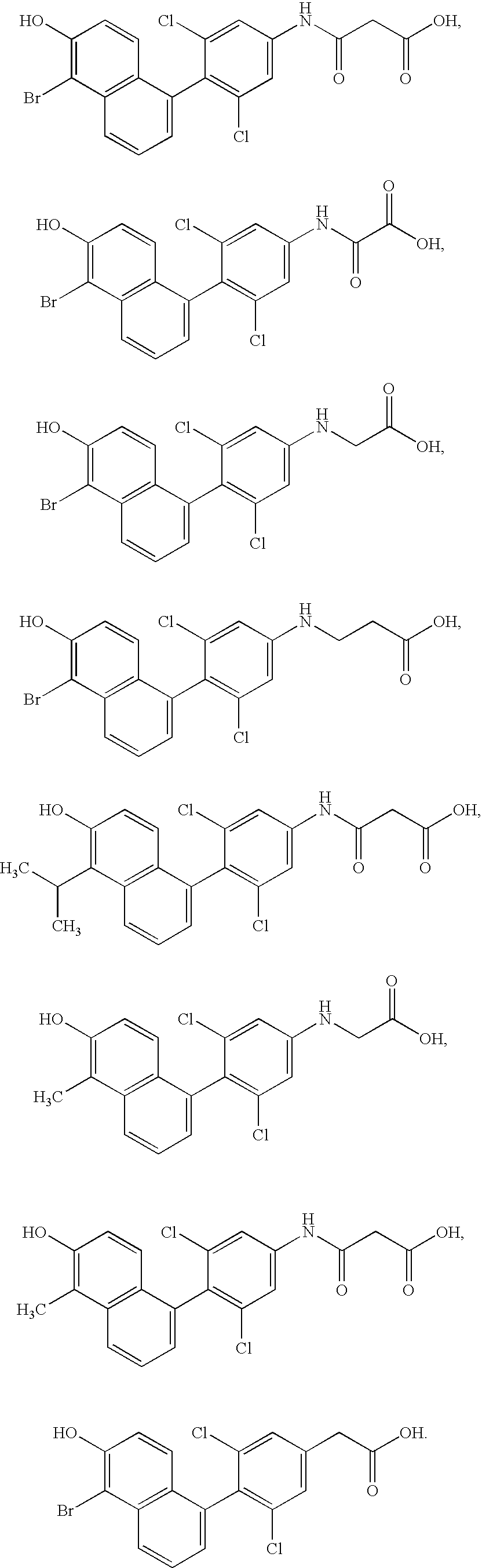
Phenyl naphthol ligands for thyroid hormone receptor
a thyroid hormone receptor and phenyl naphthol technology, applied in the field of phenyl naphthol compounds, can solve the problems of limited development of thyroid agonists and antagonists for treatment, slow discovery and development of new specific drugs for improving the treatment of hyperthyroidism and hypothyroidism, and weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-[3,5-dichloro-4-(5-bromo-6-hydroxynaphthyl)-phenyl]-3-amino-3-oxopropanoic acid
6-Methoxynaphth-1-ol (0.5 g, 2.8 mmol) and triethylamine (313 mg, 3.1 mmol) were dissolved in anhyd dichloromethane (28 mL) under a blanket of argon and cooled to −400° C. Triflic anhydride (891 mg, 3.1 mmol) was added dropwise. The reaction was warmed to −10° C. and stirred for an additional 1.5 h. Additional triflic anhydride (291 mg, 0.3 mmol) was added to complete the reaction. The reaction was quenched with water. The layers were separated and the organic layer washed with water and brine, dried over magnesium sulfate, filtered and dried in vacuo to yield 877 mg (99%) of an oil which solidifies upon standing. 1H-NMR is consistent with the proposed structure.
The triflate of Compound 1a (857 mg, 2.8 mmol), bis-picolinatodi-borane (1.07 g, 4.2 mmol) and anhyd potassium acetate (824 mg, 8.4 mmol) were placed in a one necked flask equipped with an argon inlet. The solids were suspended in anhyd DMS...
example 2
2-[3,5-dichloro-4-(5-bromo-6-hydroxynaphthyl)-phenyl]-2-amino-2-oxoacetic acid
Compound 1f (397 mg, 1.0 mmol) and triethyl amine (120 mg, 1.2 mmol) were dissolved in anhyd dichloromethane (5 mL) and cooled to 0° C. Ethyl chlorooxolate (163 mg, 1.2 mmol) was added dropwise. The reaction was warmed to room temperature and stirred overnight. The reaction was diluted with ethyl acetate and washed with water and brine, dried over anhyd magnesium sulfate, filtered and dried in vacuo. Flash chromatography (30 g silica gel, elute with 15% ethyl acetate in hexanes) provided pure product (298 mg, 60%). 1H-NMR was consistent with the proposed structure.
The ester formed by Compound 2a (60 mg, 0.12 mmol) was deprotected using the protocol described for Compound 1h yielding 25 mg, (55%) of the title compound of Example 2. 1H-NMR and mass spec were consistent with the proposed structure of Example 2.
example 3
N-[3,5-dichloro-4-(5-bromo-6-hydroxynaphthyl)-phenyl]-glycine
Compound 1f (50 mg, 0.126 mmol) was dissolved in anhyd acetonitrile (1 mL) and treated with potassium carbonate (19 mg, 0.139 mmol) and ethyl bromoacetate (23 mg, 0.139 mmol). The reaction was heated to 80° C. for 2 h. Very little reaction was evident. Additional ethyl bromoacetate (112 mg, 0.68 mmol) was added. Heating was continued overnight. The reaction was diluted with ethyl acetate and water. The layers were separated and the organic layer was washed with water and brine, dried over anhyd magnesium sulfate, filtered and evaporated in vacuo. The crude product was carried onto the next step without further purification.
The ester formed by Compound 3a (60 mg, 0.12 mmol) was deprotected using the protocol described for compound 1 hr yielding 45 mg (55%) of the title Compound of Example 3. 1H-NMR and mass spec were consistent with the proposed structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| stereoisomers | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com