Enzymatic fuel cell
a fuel cell and enzyme technology, applied in the field of batteries, can solve the problems of short operational life, high initial cost, and practical restrictions on the operation of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
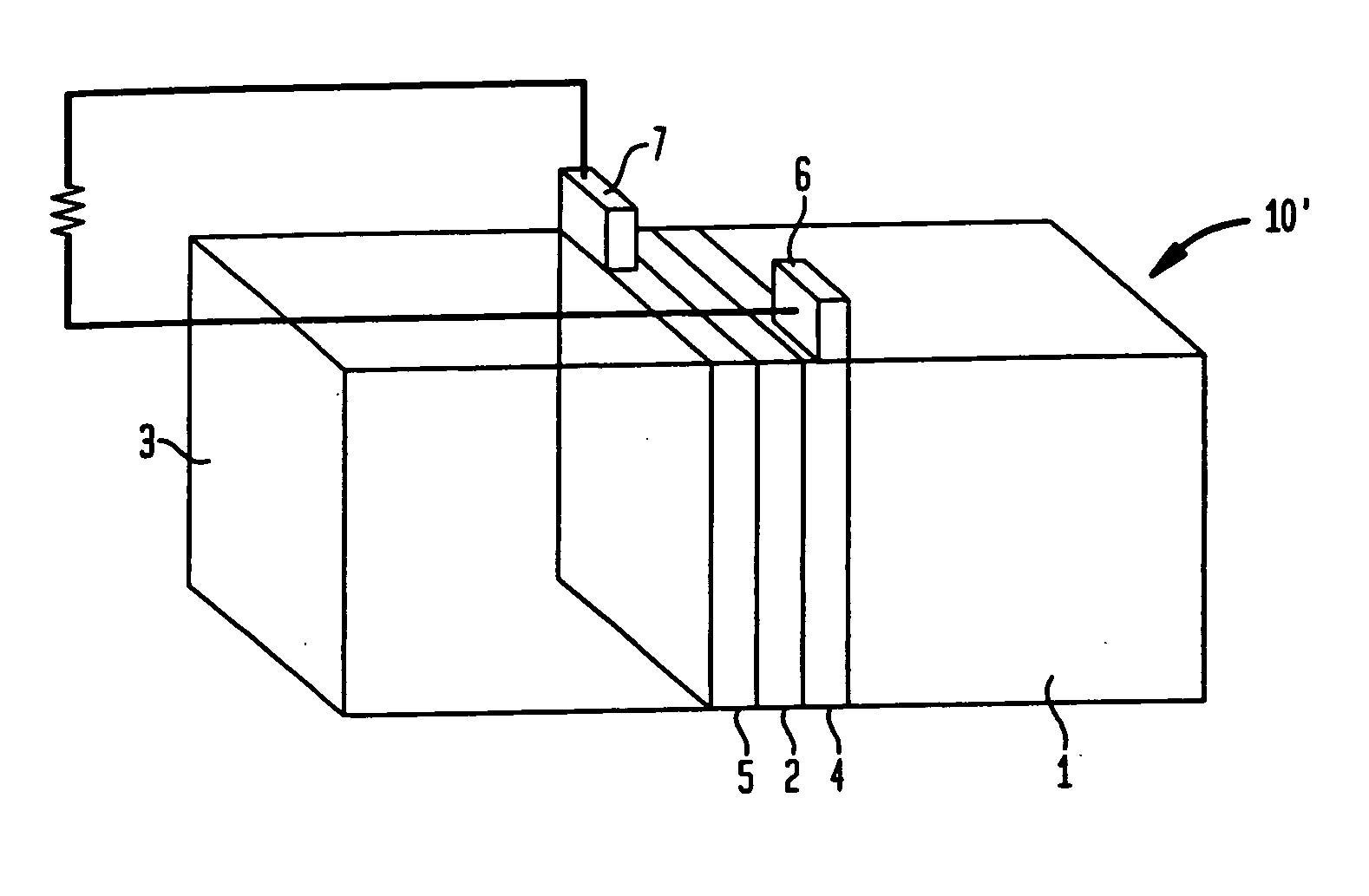
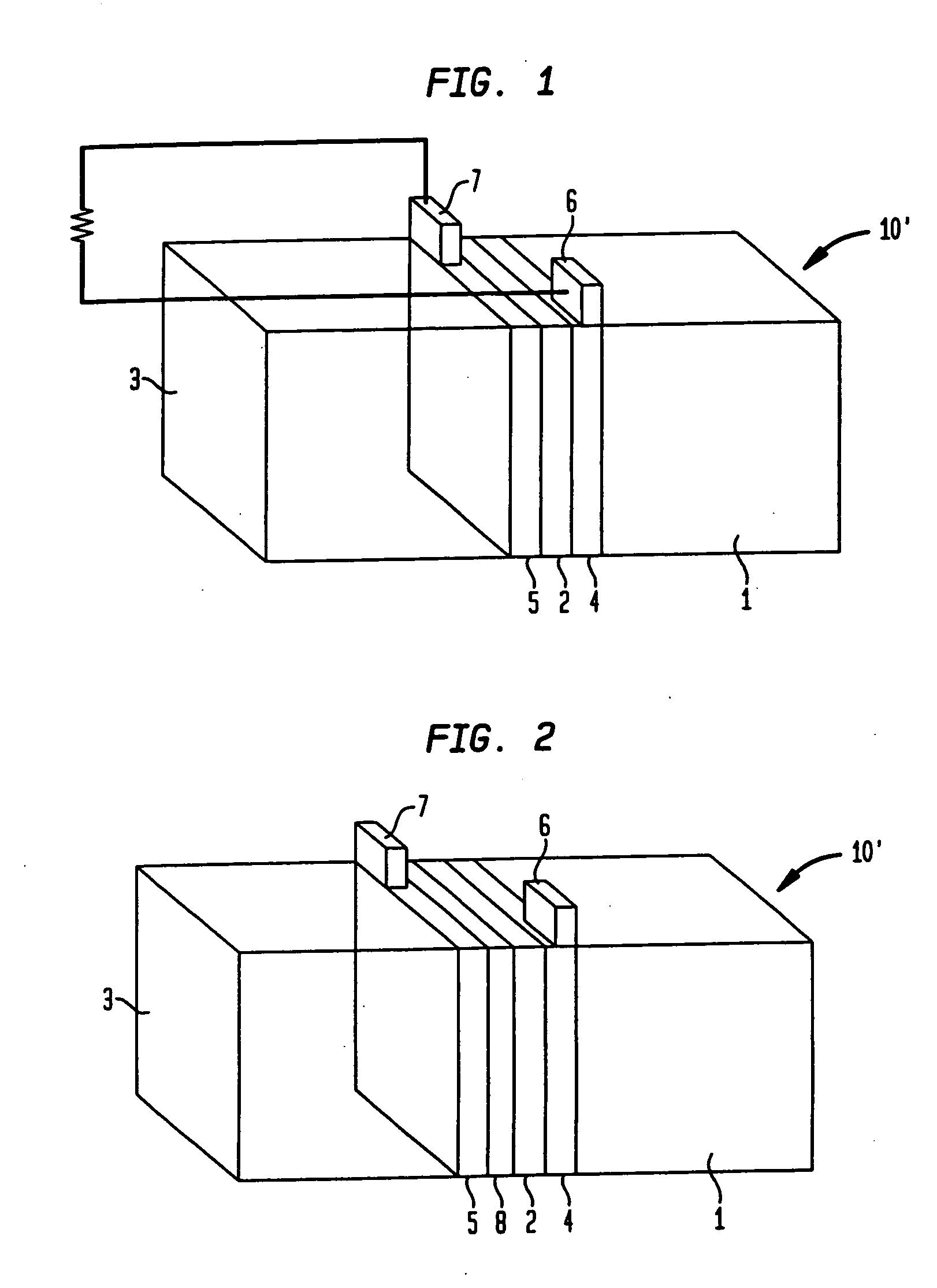
[0054] The test apparatus consisted of a 5 ml reaction vessel which held the fuel and into which copper or other electrodes were dipped. The electrodes were in turn connected to a high impedance voltmeter for open circuit voltage measurements or to a low impedance ammeter for short circuit current measurements. Various test configurations were employed to establish a baseline with which to measure performance of the cell. Testing was done by dipping electrodes in the fuel solution and measuring current and / or voltage as a function of time.
[0055] The reaction which drove the cell was the oxidation of nicotinamide-adenine dinucleotide hydride (NADH) which is catalyzed by the enzyme glucose oxidase (GOD) in the presence of glucose. This reaction yielded NAD+, a proton (H+) and 2 free electrons.
H2O+NADH=NAD++H3O++2e−
The reaction toke place at one electrode, which was a metallized plastic strip coated with the enzyme GOD. This half-reaction was coupled through an external circuit to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com