Rapidly degrading GFP-fusion proteins and methods of use
a fusion protein and rapid degradation technology, applied in the field of biochemical assays and reagents, can solve the problems of difficult to determine short-term or repetitive events, toxic to mammalian cells, unstable tbodc, etc., and achieve the effect of rapid gfp turnover, easy development of stable cell lines, and avoidance of toxic levels of gfp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of DNA Expression Vectors
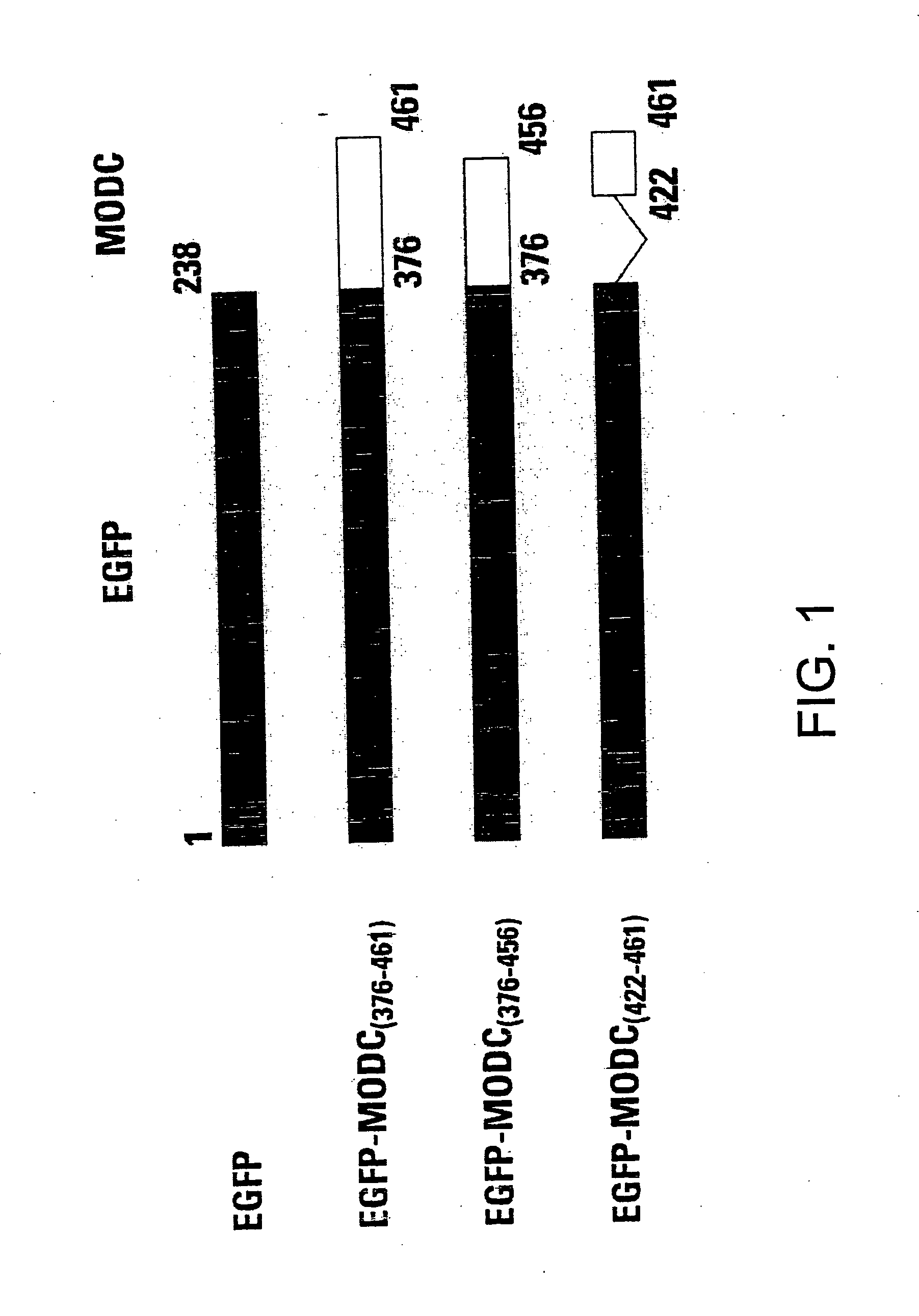
[0057] The cDNAs encoding EGFP and the C terminus of murine ODC (MODC) were amplified with pfu DNA polymerase (Stratagene, Inc., La Jolla, Calif.). EGFP was amplified with a pair of primers: 5′ incorporated with a SacII recognition sequence and 3′ with a Hind III sequence. The stop codon of EGFP was deleted from its C-terminus in order to make an open reading frame with the C terminus of murine ODC. The C terminus of murine ODC was also amplified with a pair of primers: 5′ incorporated with a Hind III recognition sequence and 3′ with an EcoRI sequence. Two amplified PCR products were ligated at the Hind III site and the fusion was cloned into pTRE expression vector, Tc-regulated expression system (Gossen M., and Bujard H. (1992) Proc. Natl. Acad. Sci. 89: 5547-5551).
[0058] Using these methods, fusion proteins of EGFP-MODC were constructed. The EGFP-MODC376-461 fusion protein included the complete C-terminus of murine ornithine decarboxylase. ...
example 2
[0059] The construct DNAs were purified and transfected into CHO K1-off cells for determination of protein degradation. CHO K1-off cells are CHO cells which were pre-transfected by a fusion protein of the tet-repressor and the herpes simplex virus VP16 gene (tTA). This pre-transfection allows expression of the gene coding for the fusion protein on a pTRE vector (Gossen and Bujard, ibid), which in turn initiates transcription by binding to a modified CMV promoter with tet-repressor binding elements. This binding can be blocked by tetracycline; hence, the expression can be controlled by tetracycline. The DNAs were introduced into these cells by CLONfectin (CLONTECH Laboratories, Inc., Palo Alto). After 24 hours, transfected cells were subject to functional analyses.
example 3
Fluorescence Analysis
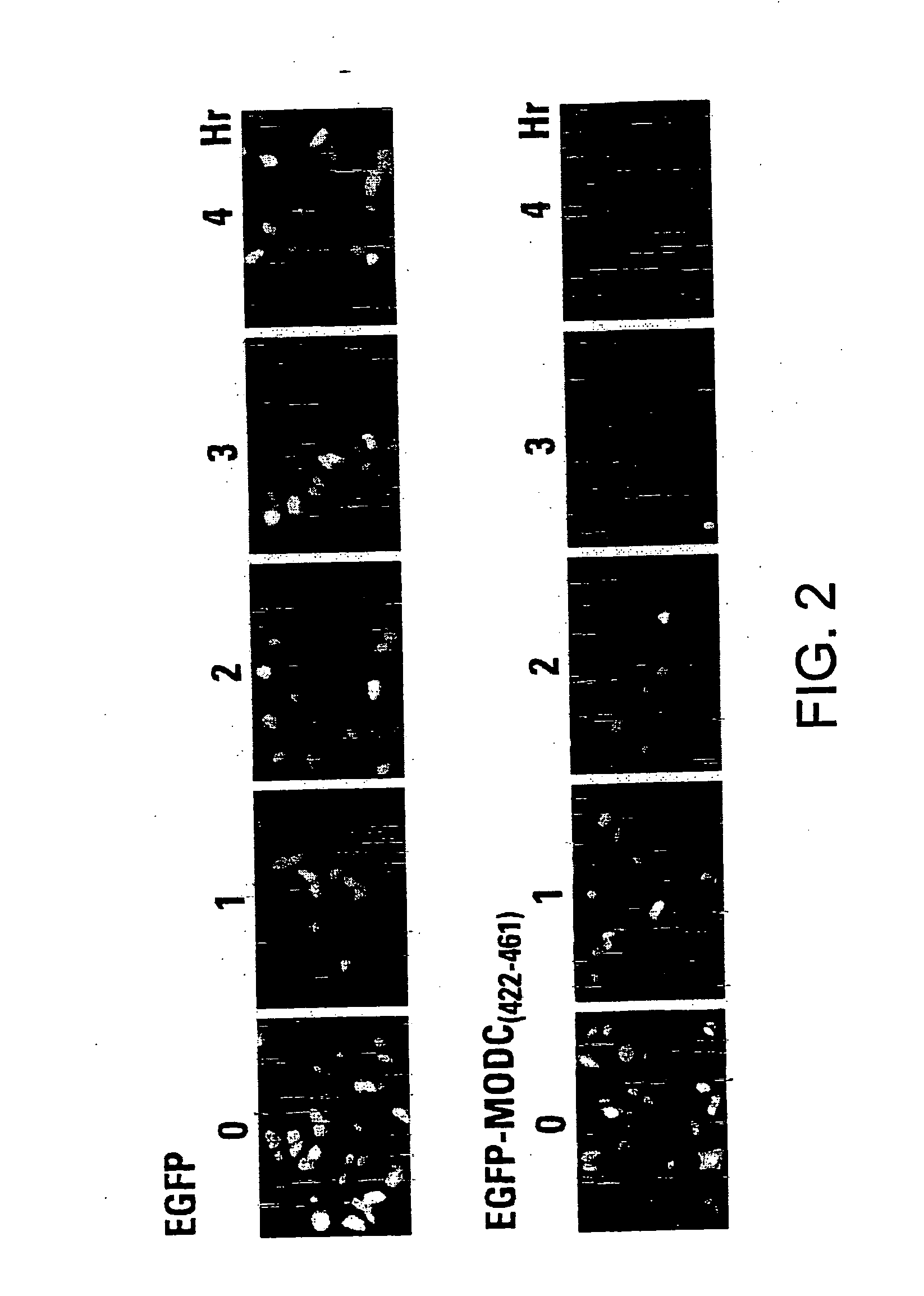
[0060] Cells were cultured on top of cover-slips to allow observation under a fluorescence microscope. After transfection, the cells were incubated at 37° C. for 24 hours on the cover-slips and then fixed with 4% paraformaldehyde for 30 minutes. The cover-slips were mounted on a glass slide for fluorescence examination with a Zeiss Axioskop Model 50 fluorescent microscope. To determine protein turnover, the cells were treated with cycloheximide at a final concentration of 100 μg / ml for variable times before paraformaldehyde fixation.
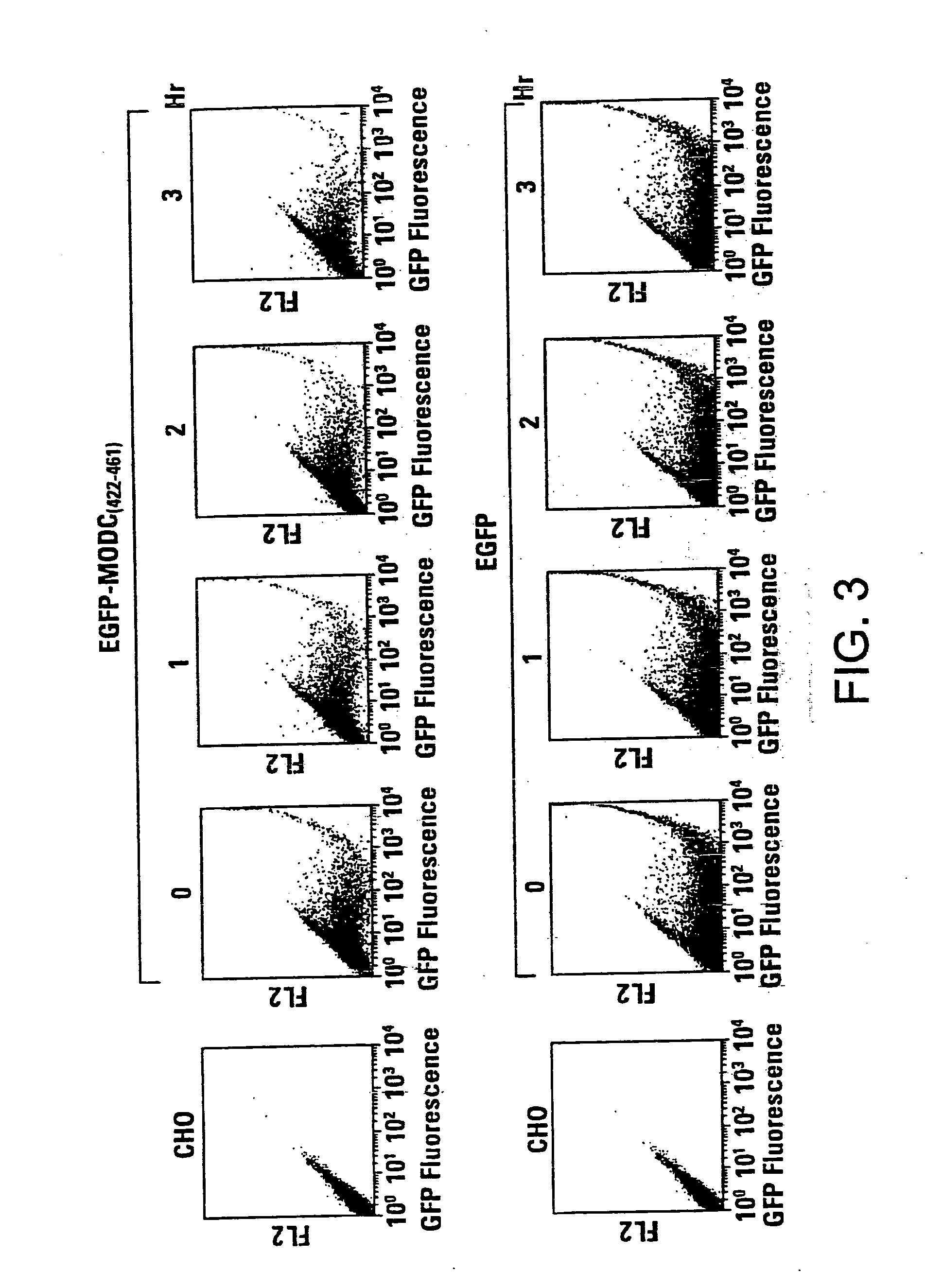
[0061] For FACS analysis, the transfected cells as well as cycloheximide-treated cells were collected by EDTA treatment and the cell pellets were resuspended in 0.5 ml of PBS. The cell suspensions were then analyzed for fluorescence intensity by FACS Calibur (Becton Dickson, Inc., San Jose, Calif.). EGFP was excited at 488 nm, and emission was detected using a 510 / 20 bandpass filter.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com