Targeted biopsy delivery system
a biopsy and delivery system technology, applied in the field of targeted biopsy delivery system, can solve the problems of difficulty in preciseness, need for additional samples to be taken, and difficulty in accurate knowledge of physicians, and achieve the effect of quick sequence extension and quick movemen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
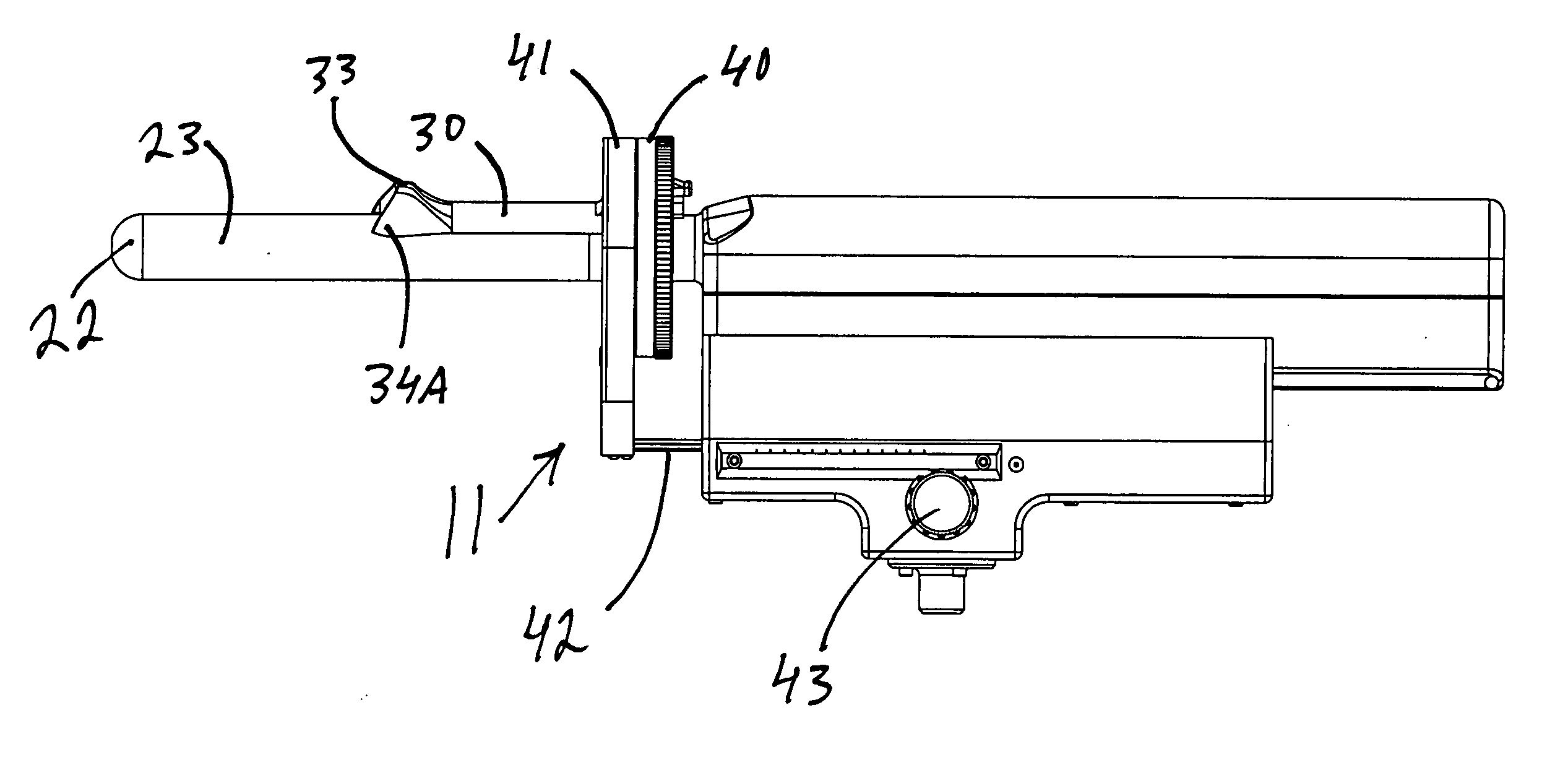
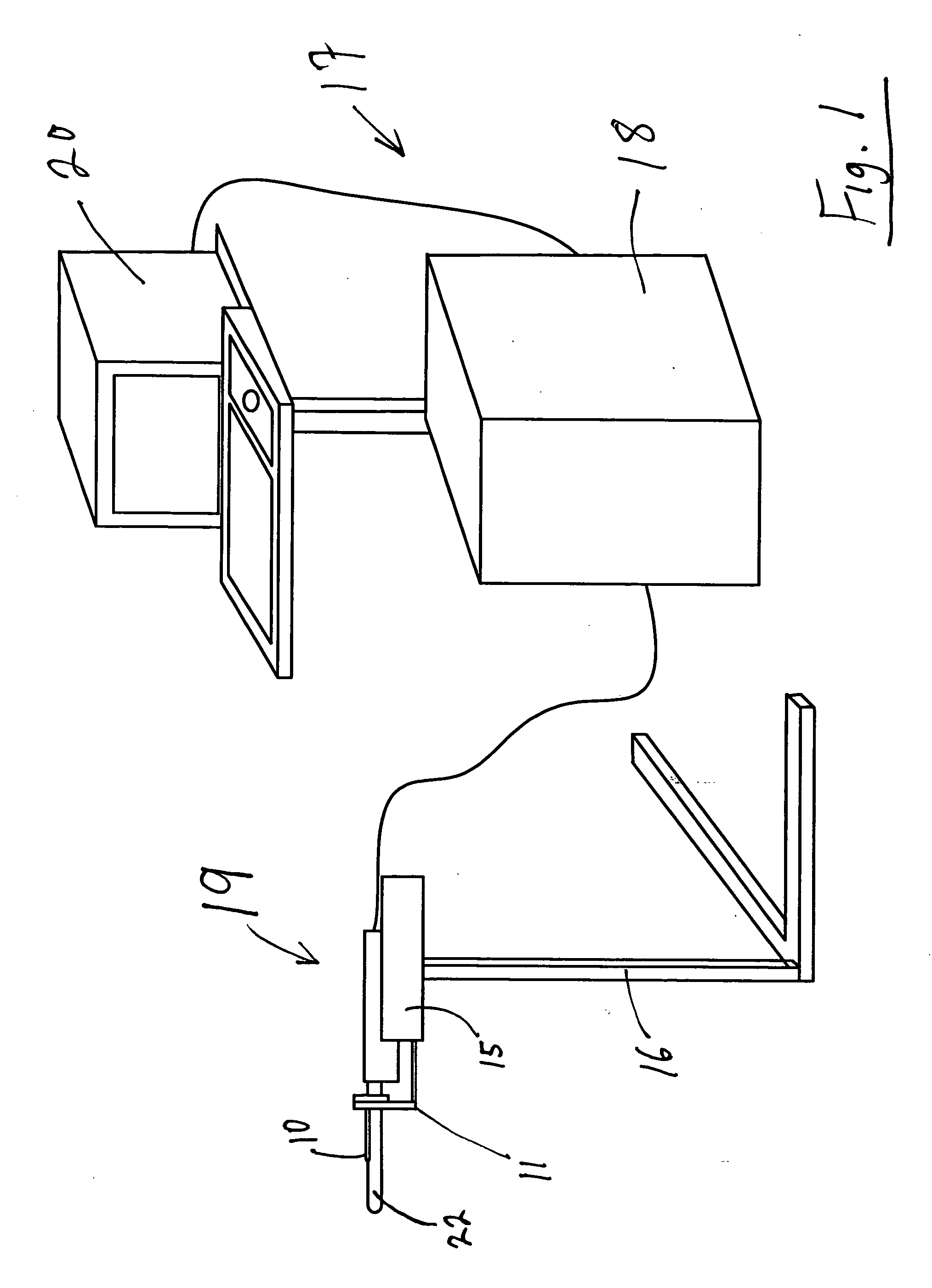
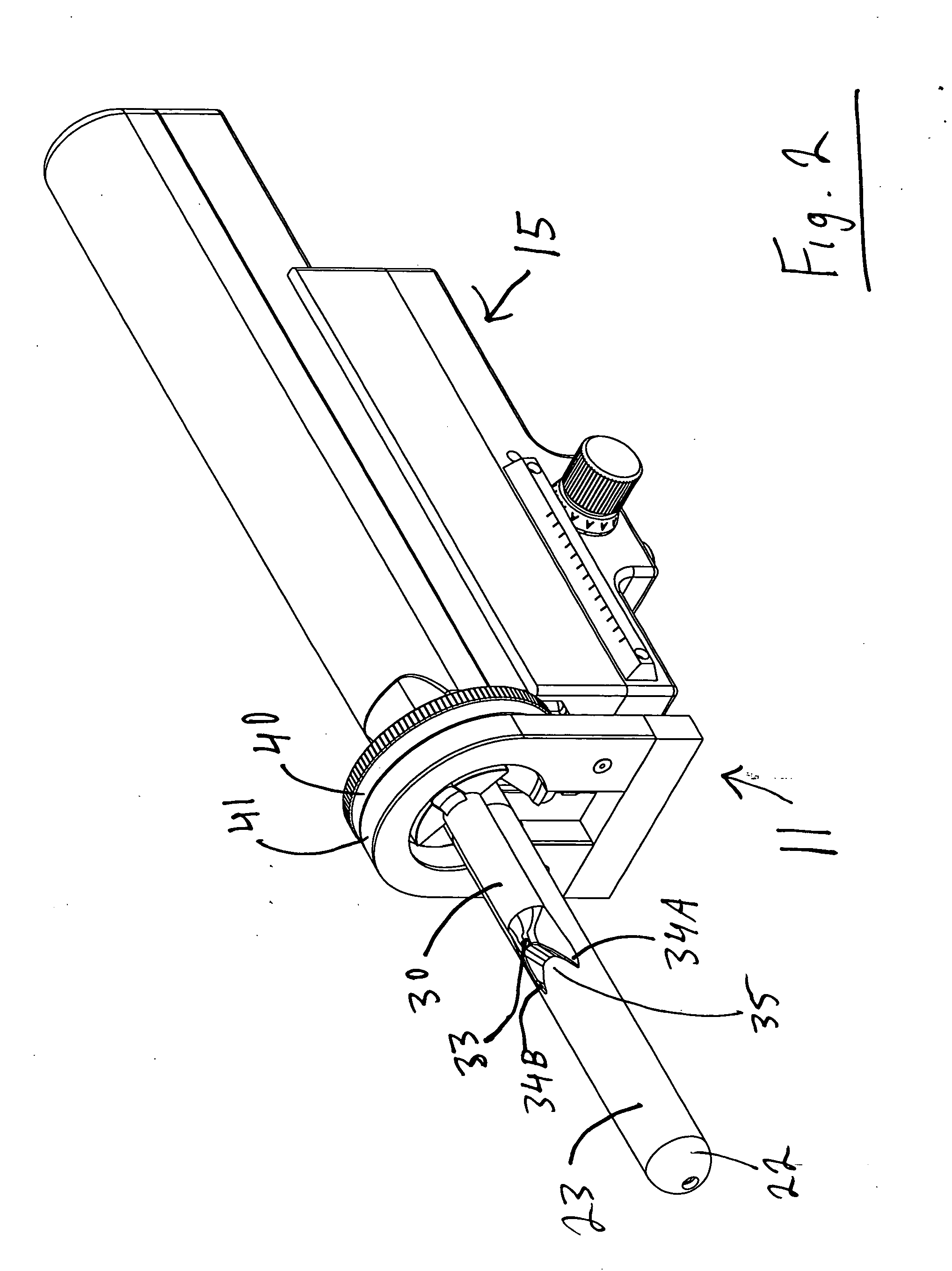
[0113] As seen in FIG. 1, the targeted biopsy system is comprised of a redirecting guide 10, positioning assembly 11, targeting software system 12 (loaded on CPU 18) and flexible needle set 13 (best seen in FIG. 20). The positioning assembly 11 is affixed to cradle 15, which is a part of stepper and stabilizer 16. Working in conjunction with the targeted biopsy system is ultrasound system 17, which is comprised of ultrasound system CPU 18, side view transrectal probe 19 and monitor 20. Side view transrectal probe is comprised of probe tip 22 and probe imaging window 23. As seen in FIGS. 2 and 7, the redirecting guide 10 consists of guide body 30, needle set channel 31, needle set insertion point 32, and needle set exit point 33, front body guide extensions 34A and 34B, imaging cutout 35. As seen in FIG. 10, needle set channel 31 may be provided with enlarged bend channel 37. As seen in FIG. 8, the redirecting guide 10 may be provided with insertable metal tube 38. In an alternative ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com