Endoglucanase gene promoter upregulated by nematodes
a technology of endoglucanase and promoter, which is applied in the field of tissue-specific gene promoters, can solve the problems of affecting the growth of endoglucanase, and affecting the growth of endoglucanase, and achieves the effect of reducing the number of nematodes and endocannase gene promoters, and reducing the number o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Plant and Nematode Culture Maintenance
[0054] Plant Material. Tobacco (Nicotiana tabacum ‘NC95’) seeds were surface sterilized with 2.5% sodium hypochlorite for five minutes, followed by several rinses with sterile water, and germinated in Petri plates containing 0.8% Noble agar (Fisher Scientific, Pittsburgh, Pa.) supplemented with Murashige and Skoog ((1962) Physiol. Plant Path. 15: 473-497) minimal media, pH 5.8 and 3% sucrose. Tobacco seedlings were grown in a controlled temperature growth chamber at 25° C. with a 14-hour photoperiod.
[0055] Nematode Cultures and Inoculations. The tobacco cyst nematode (TCN), Globodera tabacum subspecies solanacearum (Miller and Gray (1972) Nematologica 18: 404-413), and the root-knot nematode (RKN), Meloidogyne incognita Race 4 (Hartman and Sasser (1985) Identification of Meloidogyne species on the basis of differential host test and perineal—pattern morphology. In Advanced Treatise on Meloidogyne, Vol. II (Biology and Control), ed. J. N. Sasse...
example 2
In Planta Localization of TCN EGases Tissue
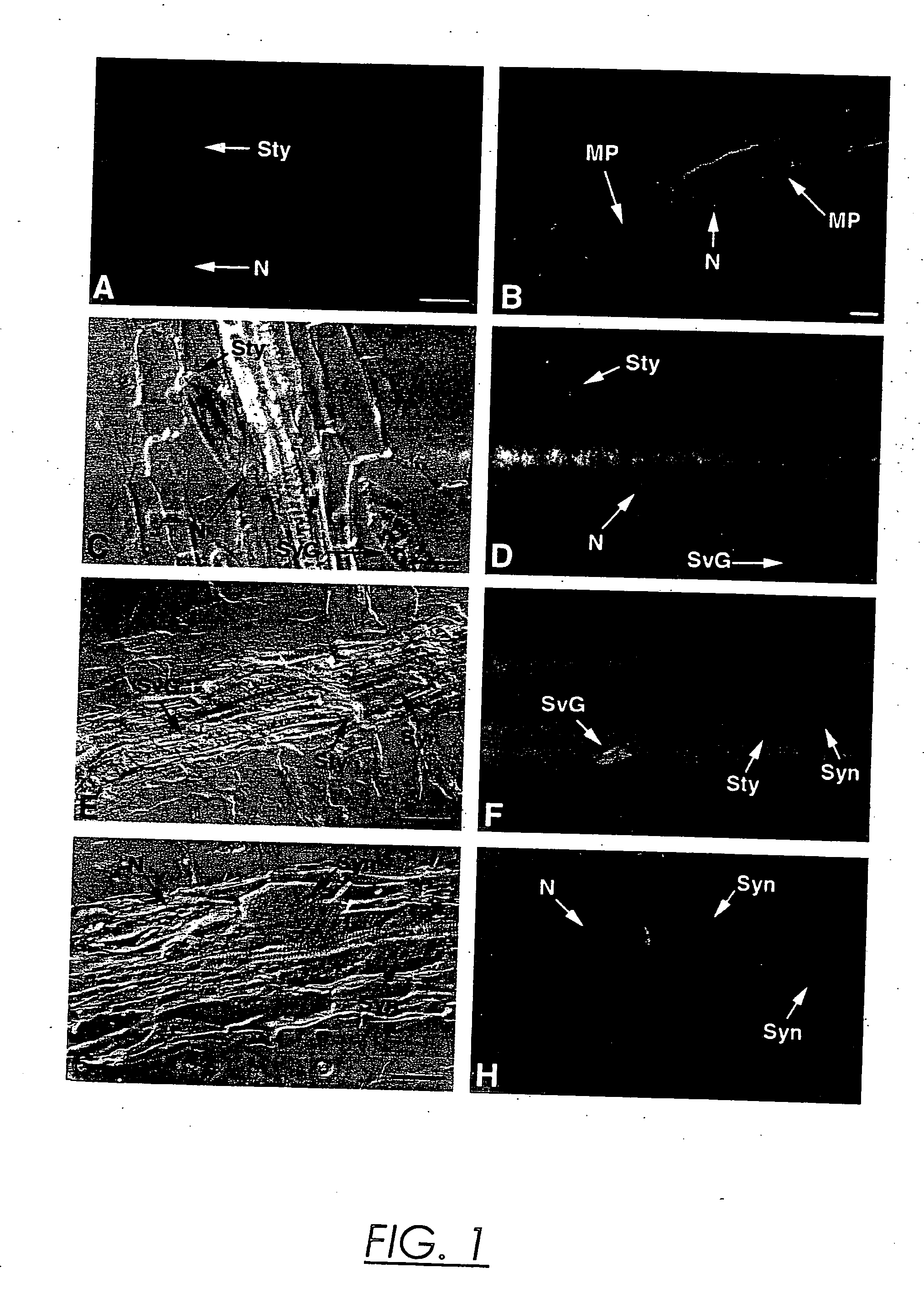
[0056] Fixation and Embedding. For immunolocalizations, TCN-infected root pieces were excised from Petri plates twenty-four to ninety-six hours after inoculation and fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS; 137 mM NaCl, 1.4 mM KH2PO4, 2.6 mM KCl, 1.8 mM Na2HPO4, pH 7.4) for three hours at room temperature. After two 15 minute washes with PBS, the fixed root pieces were dehydrated in a graded ethanol series (30%, 60%, 70%, 85%, 95%, 100%, 15 min. each) and then incubated sequentially in ethanol: Histoclear (National Diagnostics, Atlanta, Ga.) 75:25, 50:50, 25:75 for 10 minutes each. After two 15 minutes incubations in 100% Histoclear, the root pieces were transferred to molten Paraplast plus (Fisher Scientific, Pittsburgh, Pa.) at 60° C. for two hours and embedded in blocks. For in situ mRNA localizations, nematode-infected tobacco root pieces were dissected from Petri plates 7-9 days or 12-14 days after infection and...
example 3
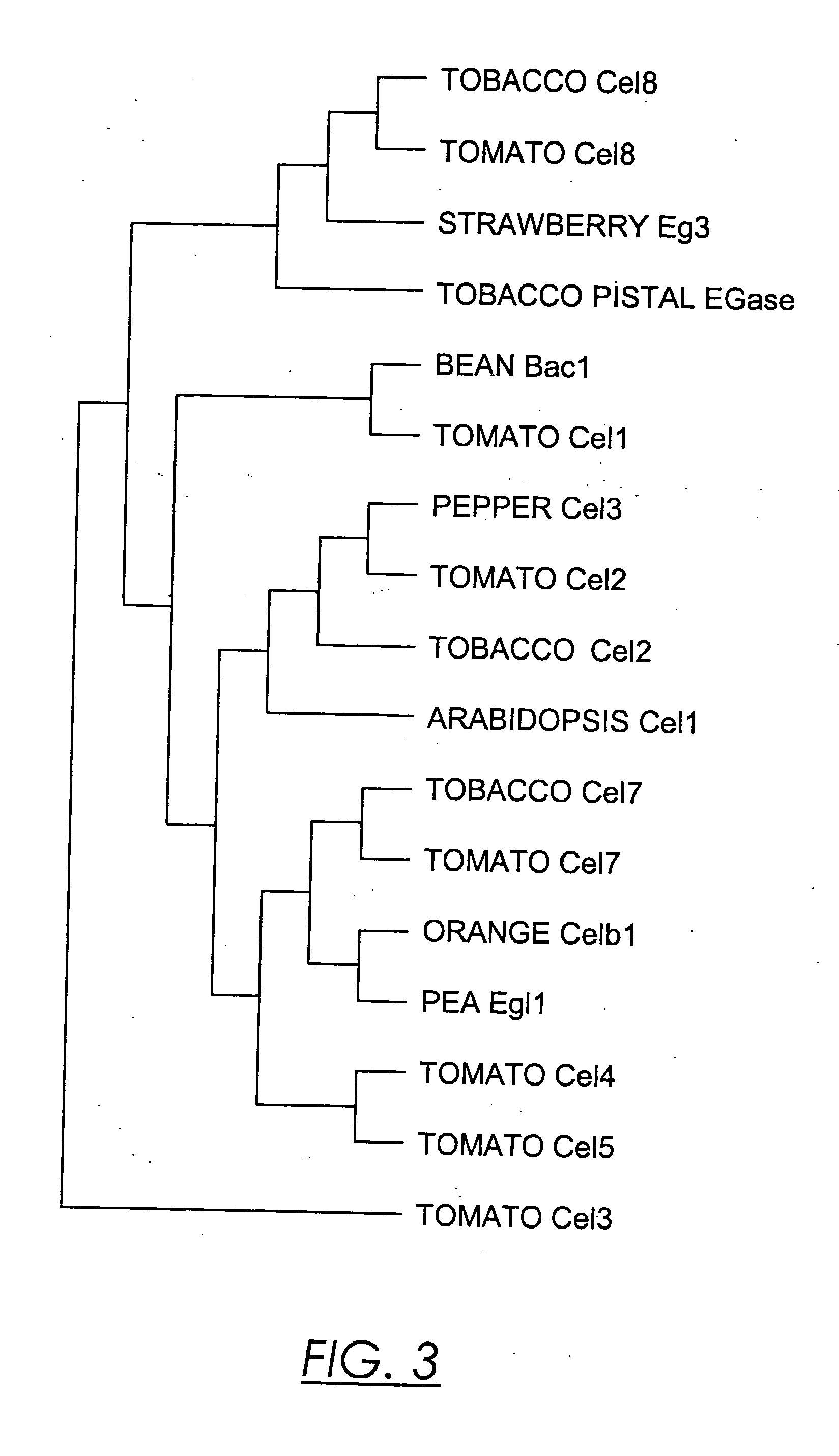
Isolation and Sequence Characterization of Tobacco EGases
[0060] To isolate poly A(+) RNA, 5 cm of infected or noninfected tobacco root pieces (excluding root tips) were excised from Petri plates and ground in a small glass homogenizer in 250 μl lysis-binding buffer (100 mM Tris-HCl, pH 7.5, 500 mM LiCl, 10 mM EDTA, pH 8.0, 5 mM dithiothreitol, 1% LiDS; Dynal, Lake Success, N.Y.). After lysis, the homogenate was centrifuged for one minute at 13,000×g and the supernatant was transferred to a clean tube. Twenty-five microliters of Dynal magnetic oligo-(dT)25 beads equilibrated with lysis-binding buffer were added to the supernatant and placed on a rotator for 5 minutes to allow the mRNA to anneal to the beads. Using a magnetic stand the beads were washed twice in washing buffer with LiDS (10 mM Tris-HCl, pH 7.5, 0.15 M LiCl, 1 mM EDTA, 0.1% LiDS) and three times in washing buffer without LiDS. For first strand cDNA synthesis, the beads were washed several times in IX first strand buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com