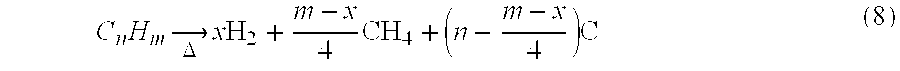Pyrolysis-based fuel processing method and apparatus
a fuel processing method and fuel technology, applied in the direction of combustible gas production, hydrogen separation using solid contact, climate sustainability, etc., can solve the problems of not being widely used in methanol, not readily available in most vehicles, and raising concerns about passengers' safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
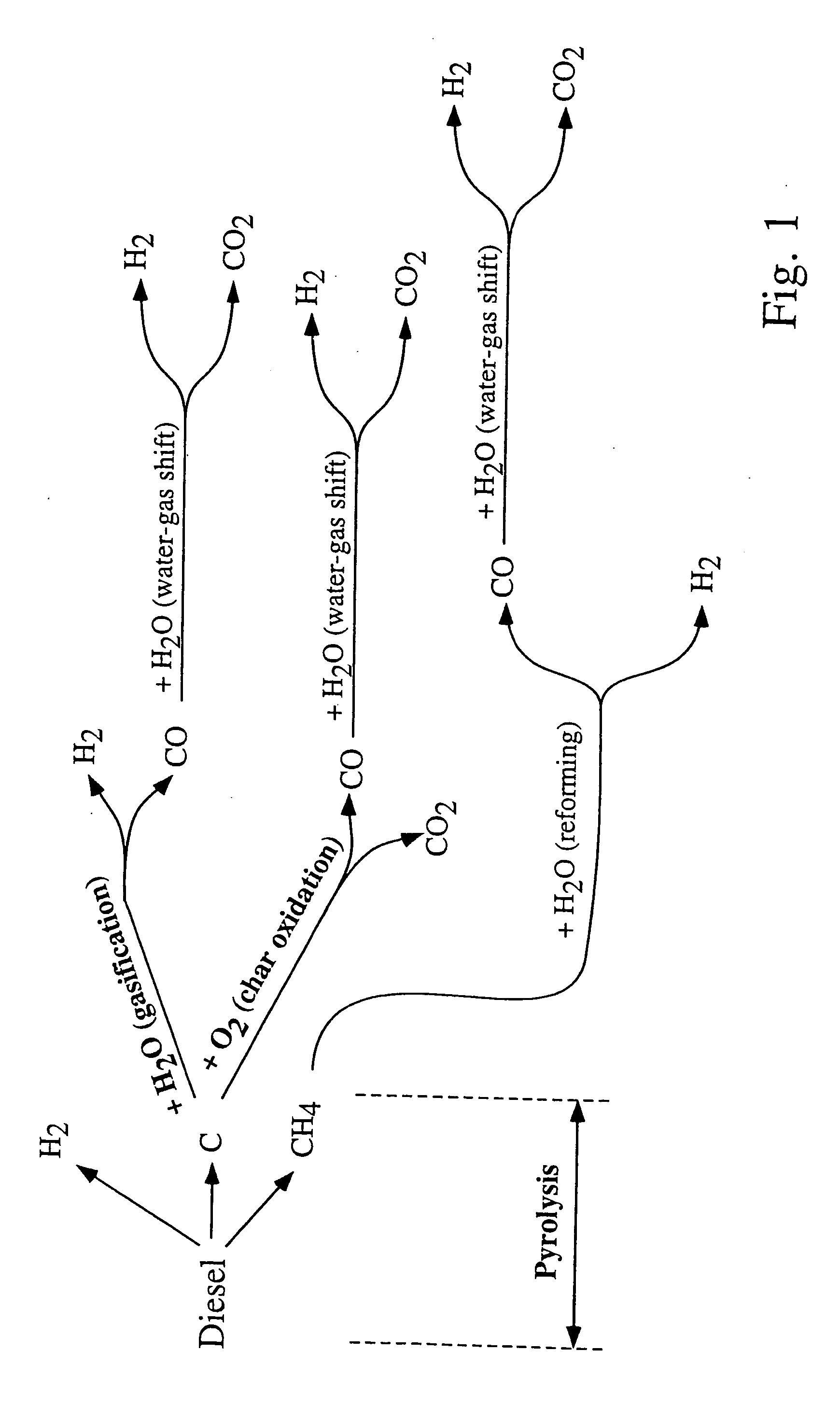
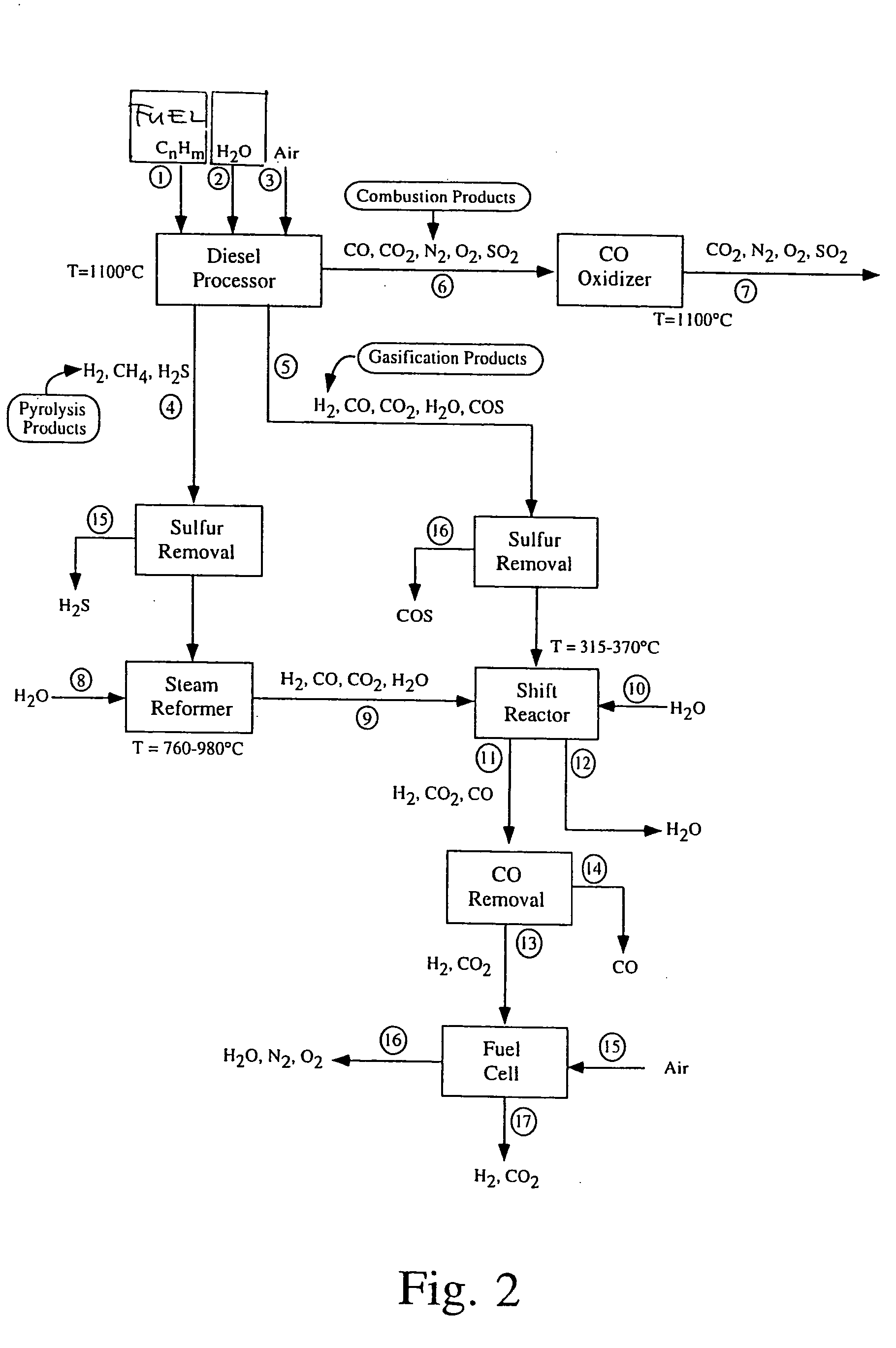
[0026] The fuel-conversion process is divided into several phases that preferably take place in the same reactor. The reactor mass, including packing (which will preferably comprise a catalyst bed), is used as a heat-transfer medium in such a way that the heat required by endothermic reactions is provided from preceding exothermic cycles. Thus, the reactor mass, which may comprise the reactor walls, catalyst bed, refractory liners, any suitable packing that increases the thermal capacity of the system, etc., constitutes the means for absorption and release of heat. The operation of the fuel processor is described in steps a-d below. FIG. 1 provides an overview of reaction pathways. [0027] At a cold start (not included in FIG. 1), the fuel is burned with air within the reactor volume until the maximum temperature of the reactor mass Tmax. is reached. The exhaust gases from this cycle are discarded. As an alternative, a rapid start-up could be achieved by the initial heating of the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com