Cardiac drug delivery system and method of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
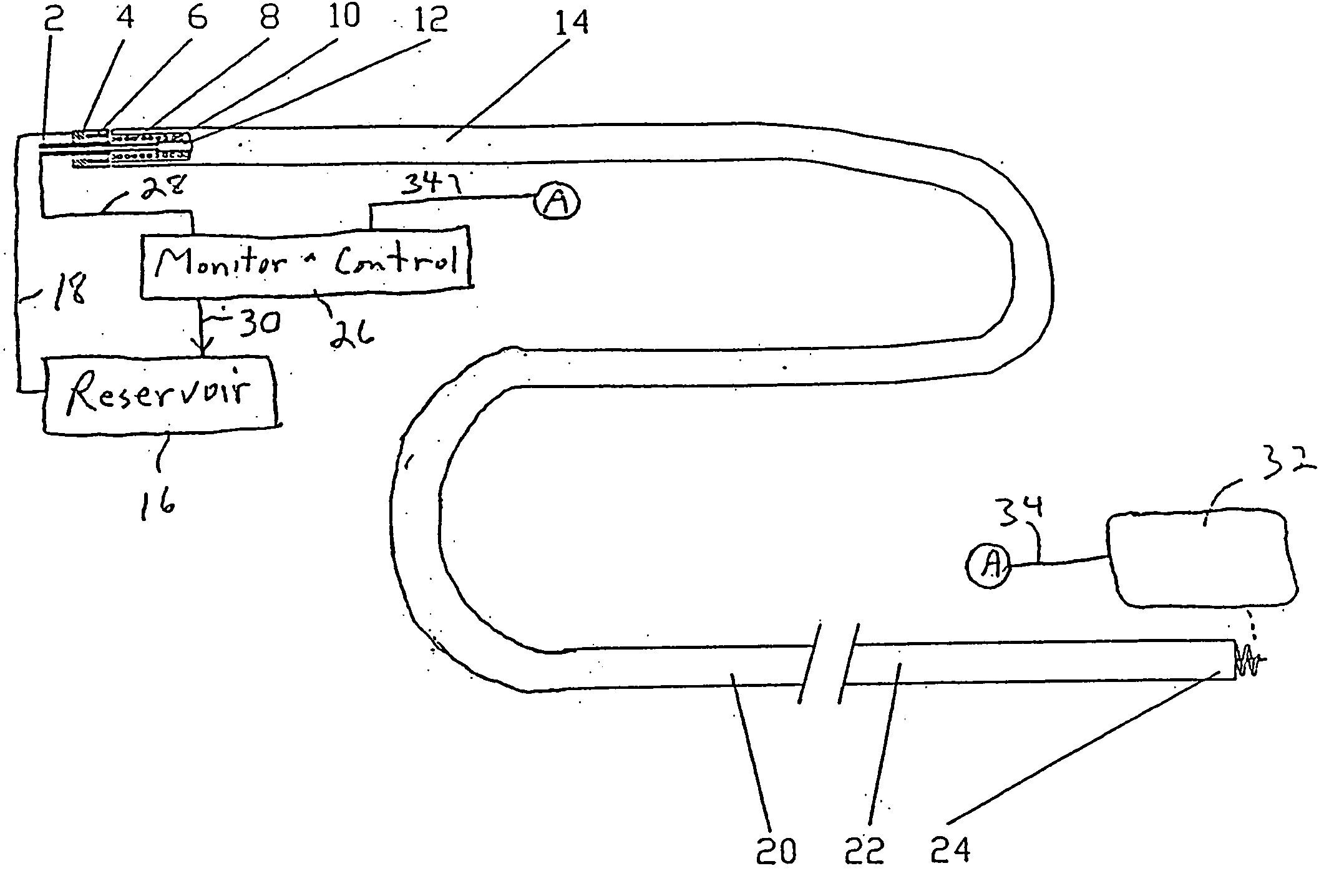
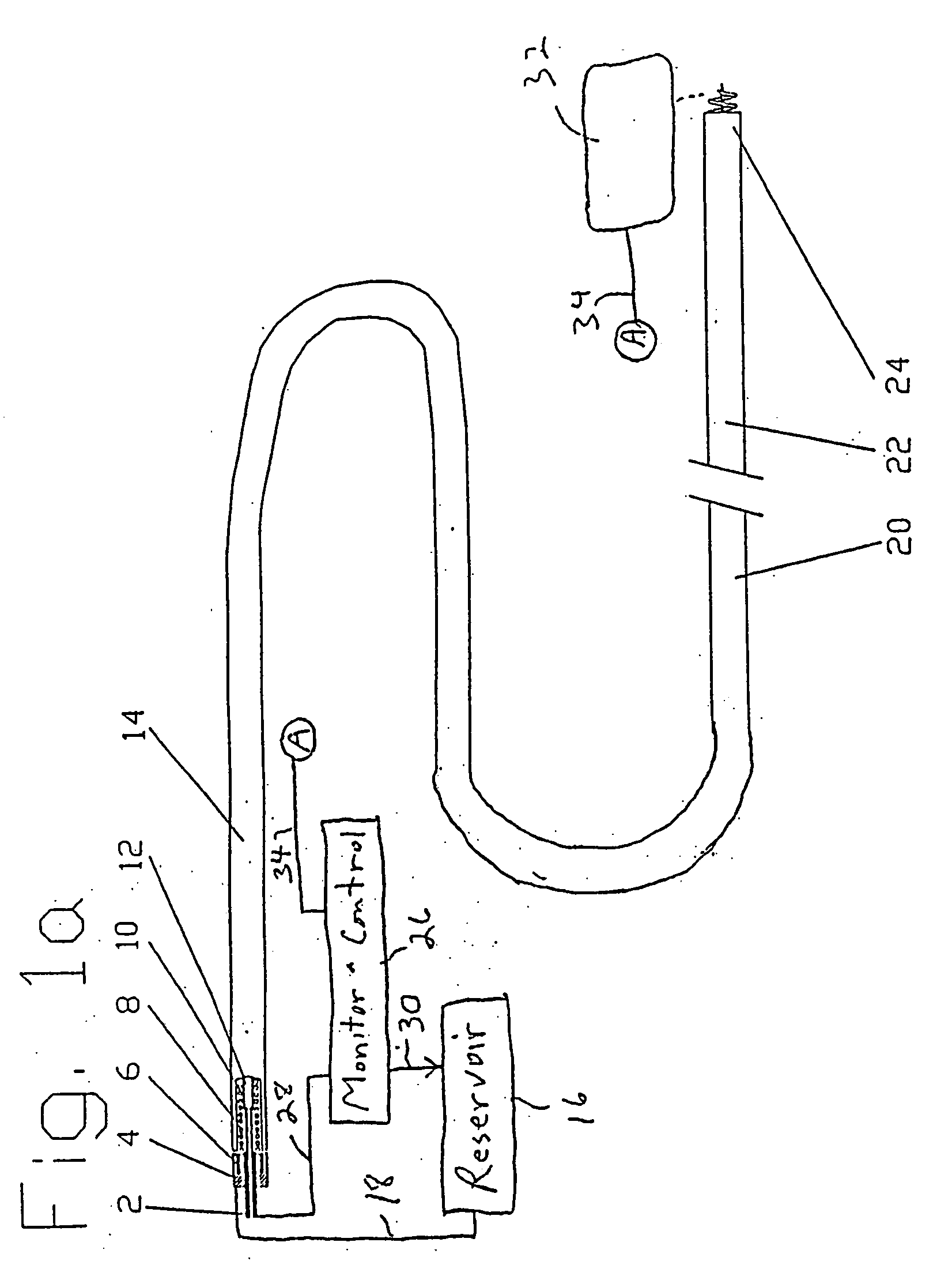
[0043] New concepts for delivering agents for the treatment of heart failure, ischemia, arrhythmias, and restenosis are disclosed. The main embodiments consist of transvenous or transarterial catheter delivery techniques for delivering agents directly to a chosen site within the heart at a depth within the heart tissue. Hollow helical delivery devices, needle delivery devices, and implantable controlled release matrices may be inserted such that metabolic agents, anti ischemic agents, growth factors, antiarrhythmic agents, anti-inflammatory agents anti-proliferative agents, gene therapy preparations, and combinations of these agents may be delivered directly to the tissue that can benefit most from these agents.
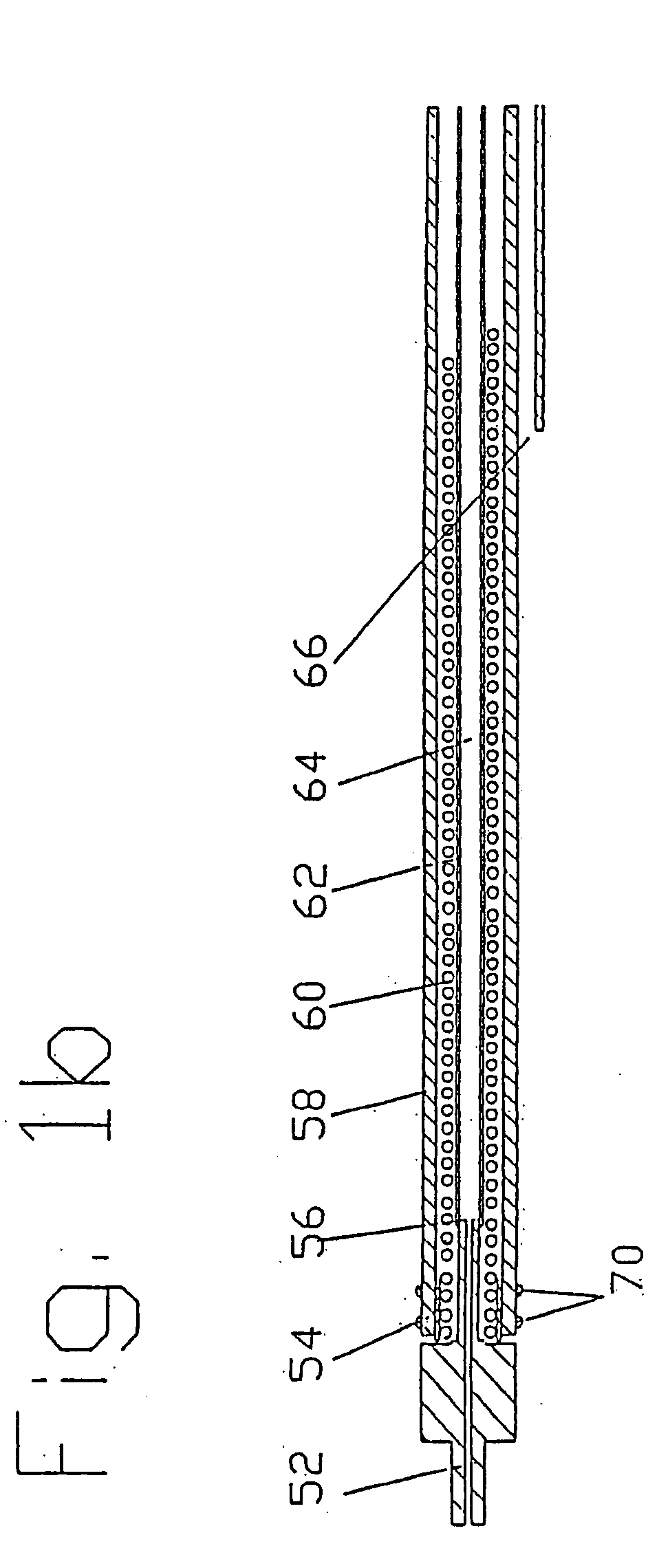
[0044] These drug delivery structures may be made from different materials depending upon whether the device is to be used chronically or acutely. For example, metal components in the preferred implantable embodiments, formed of a Platinum Iridium alloy consisting of ninety ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Flexibility | aaaaa | aaaaa |
| Depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com