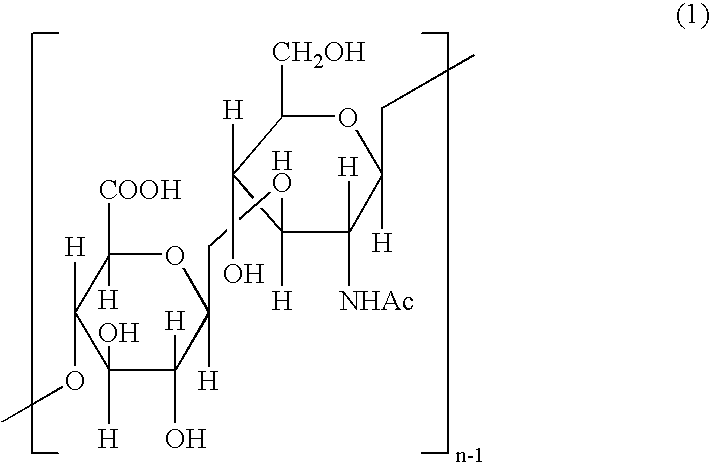
Use of hyaluronic acid polymers for mucosal delivery of vaccine and adjuvants
a technology of hyaluronic acid and polymer, which is applied in the field of bioadhesive polymer systems, can solve the problems of few bioadhesive delivery systems commercially available, local irritation, and inability to duplicate the performance of these bioadhesives in vivo, and achieves the effects of enhancing the immunogenicity of the antigen coadministered, reducing the rate of mucociliary clearance, and effective methods of elici
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Use of HYAFF Formulations Including Influenza Antigen
[0089] Placebo (Blank) microparticles of HYAFF11 polymer, approximately 100% esterified with benzyl alcohol, were supplied by Fidia Advanced Biopolymers Srl (Abano Terme, Italy). The average size of these microparticles was about 8 microns (with a portion of the size distribution being less than 1 micron and a portion above 10 microns), as determined by a Malvem Mastersizer Instrument.
[0090] In order to achieve a dose of 10 μg influenza antigen H3N2 (“HA”) (Chiron Vaccines, Sienna, Italy) and 10-25 μg LT-K63 (International Publication No. WO 93 / 13202), a 1% w / w loading of HA / LT-K63 to microparticles was targeted. To do so, 1 mg of HA and 1 mg of LT-K63, in 84 mM Na2HPO4, 11 mM KH2PO4, 82 mM NaCl, was incubated with 100 mg of blank microspheres in PBS at 4° C. for three hours. The suspension was then frozen at −80° C. and freeze-dried overnight.
[0091] Actual antigen / adjuvant load was confirmed by hydrolyzing the ...
example 2
Preparation and Use of ACP Formulations Including Influenza Antigen
[0095] Auto-crosslinked polysaccharide (ACP) hyaluronic acid gel was obtained from Fidia Advanced Biopolymers Srl (Abano Terme, Italy) and used as shipped. To the gel was added 10 μg of HA and 10-25 μg LT-K63, in an aqueous solution to make a gel-to-water ratio of 1:30.
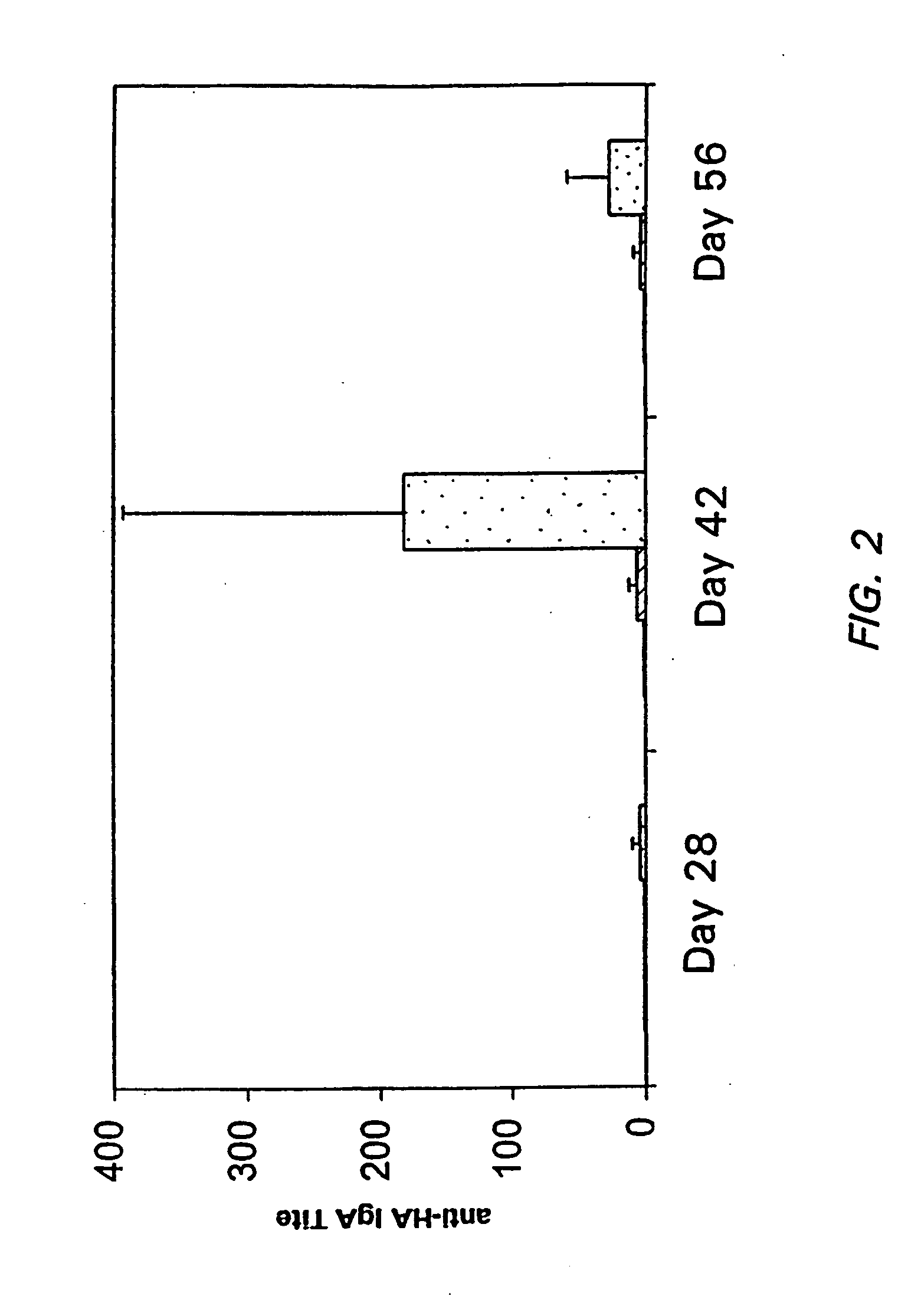
[0096] 50 μl of the viscous solution was administered intranasally using a micropipette to three groups of Balb / C mice each with five animals,.as shown in Table 2 below. Formulations were administered within 60 minutes of preparation. Animals were boosted 28 days later, bled on day 42 and anti-HA titers determined by ELISA. IgA titers from a nasal wash were also assayed.
[0097] As shown in Table 2, animals administered antigen in combination with ACP and adjuvant had higher antibody titers than those administered antigen alone.
TABLE 2Serum anti-HAIgA Titers in theGroupsFormulationELISA TitersNasal Wash1ACP (1:30) +449 + / − 84 1836 + / − 630LT-K63 (25 ...
example 3
Comparison of HYAFF and ACP Formulations
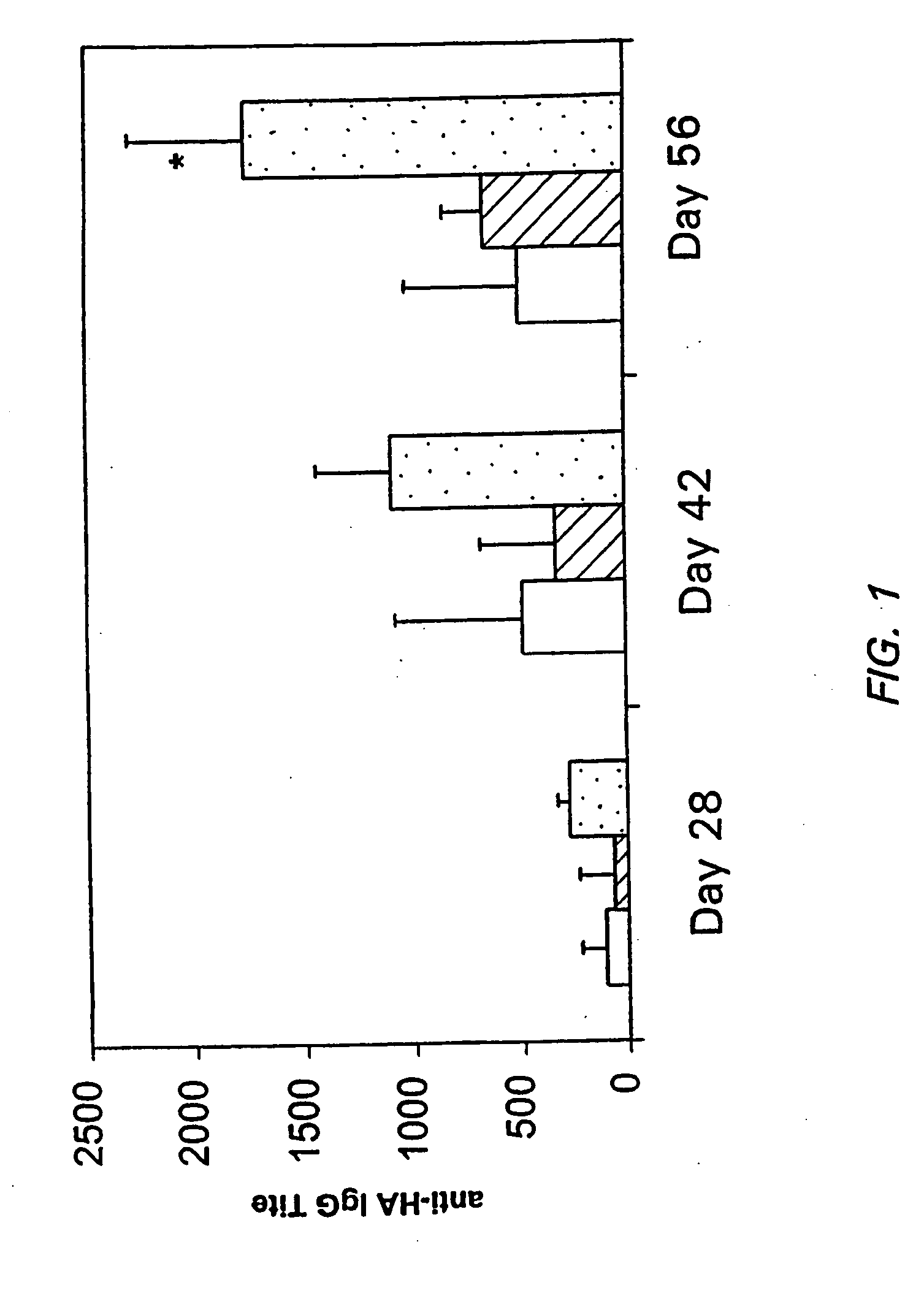
[0099] The immunogenicity of the HA antigen in HYAFF and ACP gel was evaluated in micro pigs (Yucatan). 12 pigs were divided into three groups of four pigs each, as shown in Table 4. In order to achieve the proper dose, pigs were administered intranasally using a gauge 16 Teflon catheter, 500 μl of the ACP formulation or 50 mg of the HYAFF formulation. Control pigs were given 500 μL of antigen alone. Pigs were boosted at 28 days and sera collected and assayed for anti-HA serum IgG levels using an ELISA.
[0100] As can be seen in Table 4, groups of pigs administered antigen with HYAFF or ACP both had higher titers than pigs administered antigen alone, with pigs administered the HYAFF formulations having the highest titers.
TABLE 4Serum Anti-HAGroupsFormulationELISA Titers1LT-R72 (100 μg) + HA (100 μg) 505 + / − 1832LT-R72 (100 μg) + HA (100 μg) − HYAFF1919 + / − 6023LT-R72 (100 μg) + HA (100 μg) + ACP 871 + / − 155
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com