Systems and methods for generating hydrogen from hycrocarbon fuels
a technology of hydrocarbon fuel and system, which is applied in the direction of hydrogen separation using solid contact, chemical apparatus and processes, chemical/physical processes, etc., can solve the problems of metal sulfides being less stable at high reforming temperatures, using sulfur-laden hydrocarbon fuels, and catalysts becoming ineffective by coke accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example i
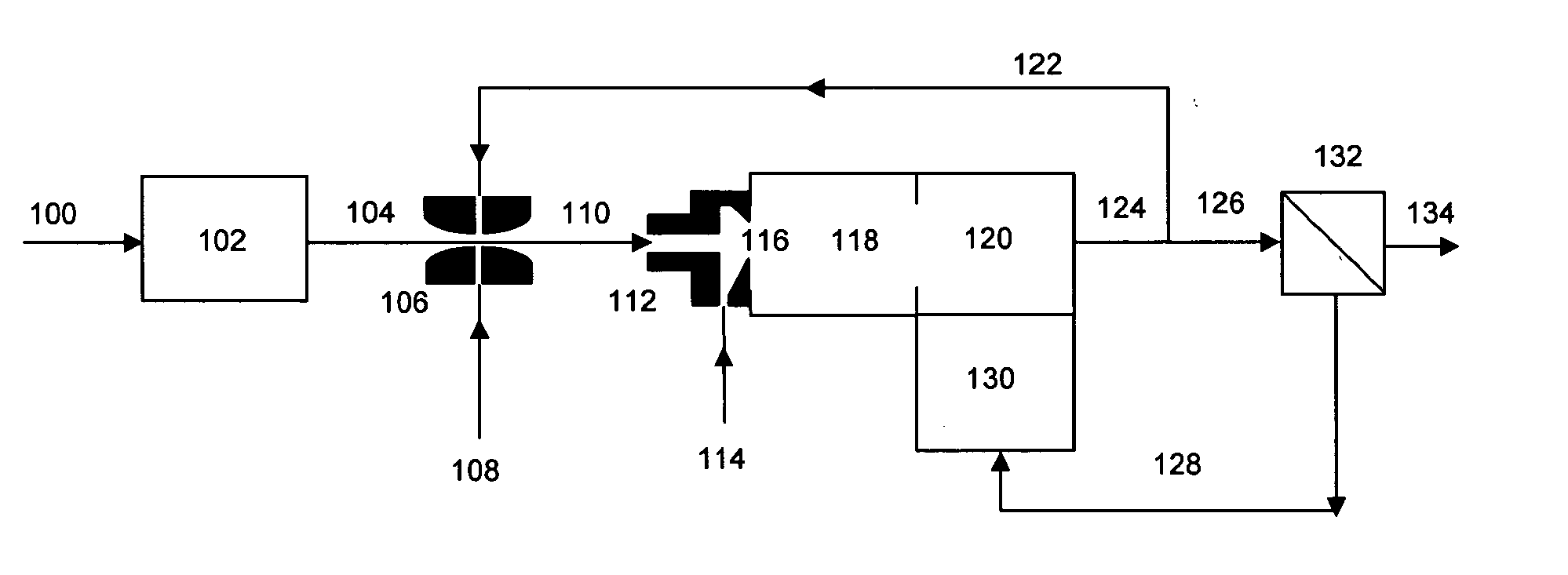
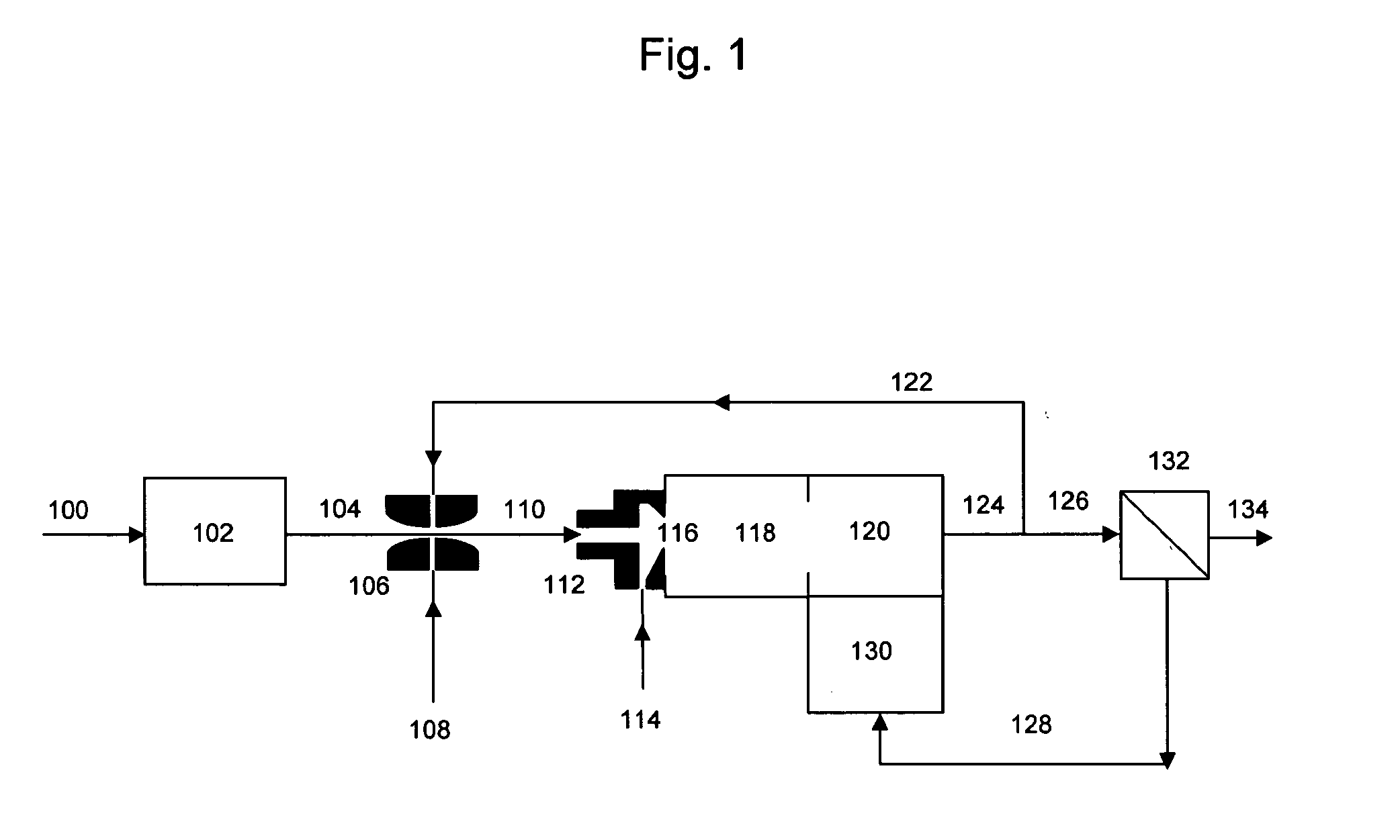
[0035] The system of FIG. 1 is operated with a logistic diesel fuel as feed. The molecular weight of the fuel is 220 gm / mole, the molecules contain on average 16 carbon atoms each, and their hydrogen to carbon ratio is 1.8. The sulfur content of the fuel is one percent by weight. The flow rate of stream 100 is adjusted relative to stream 114 so that the number of moles of water in stream 104 is three times the number of atoms of carbon in stream 114. The fuel is completely converted to hydrogen and carbon oxides in reaction zones 118 and 120. The flow rate of stream 122 is adjusted to be equal to the flow rate of stream 126. Stream 116 contains then 340 moles of hydrogen per atom of sulfur, and 4 moles of water per atom of carbon.
[0036] The temperature of reaction zone 118 is set to 500° C. The active metal in the catalyst is iridium. According to the teachings of U.S. Pat. No. 3,441,395 of Dent et al., no coke is formed when the number of moles of water per carbon atom fed to the ...
example ii
[0037] The system of FIG. 1 is operated with a low-sulfur diesel fuel as feed. The molecular weight of the fuel is 220 gm / mole, the molecules contain on average 16 carbon atoms each, and their hydrogen to carbon ratio is 1.8. The sulfur content of the fuel is fifteen parts per million by weight. The flow rate of stream 100 is adjusted relative to stream 114 so that the number of moles of water in stream 104 is three times the number of atoms of carbon in stream 114. The fuel is completely converted to hydrogen and carbon oxides in reaction zones 118 and 120. The flow rate of stream 122 is adjusted to be equal to one percent the flow rate of stream 126. Stream 116 contains then 4400 moles of hydrogen per atom of sulfur, and 3 moles of water per atom of carbon. The temperature of reaction zone 118 is set to 500° C. The active metal in the catalyst is rhodium. Predominance diagrams indicate that for a Rh catalyst the metallic form predominate over the metal sulfide when the catalyst op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com