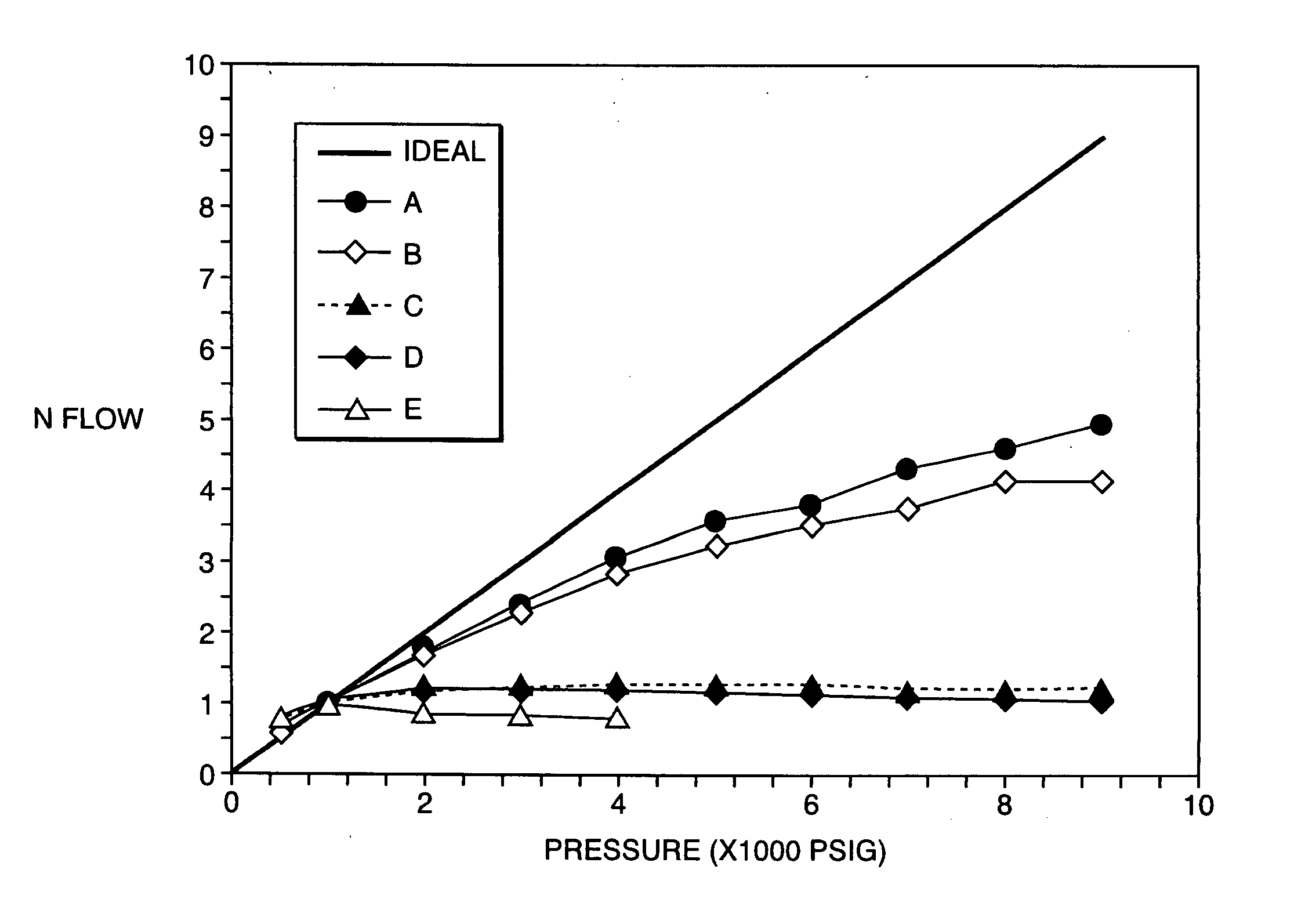
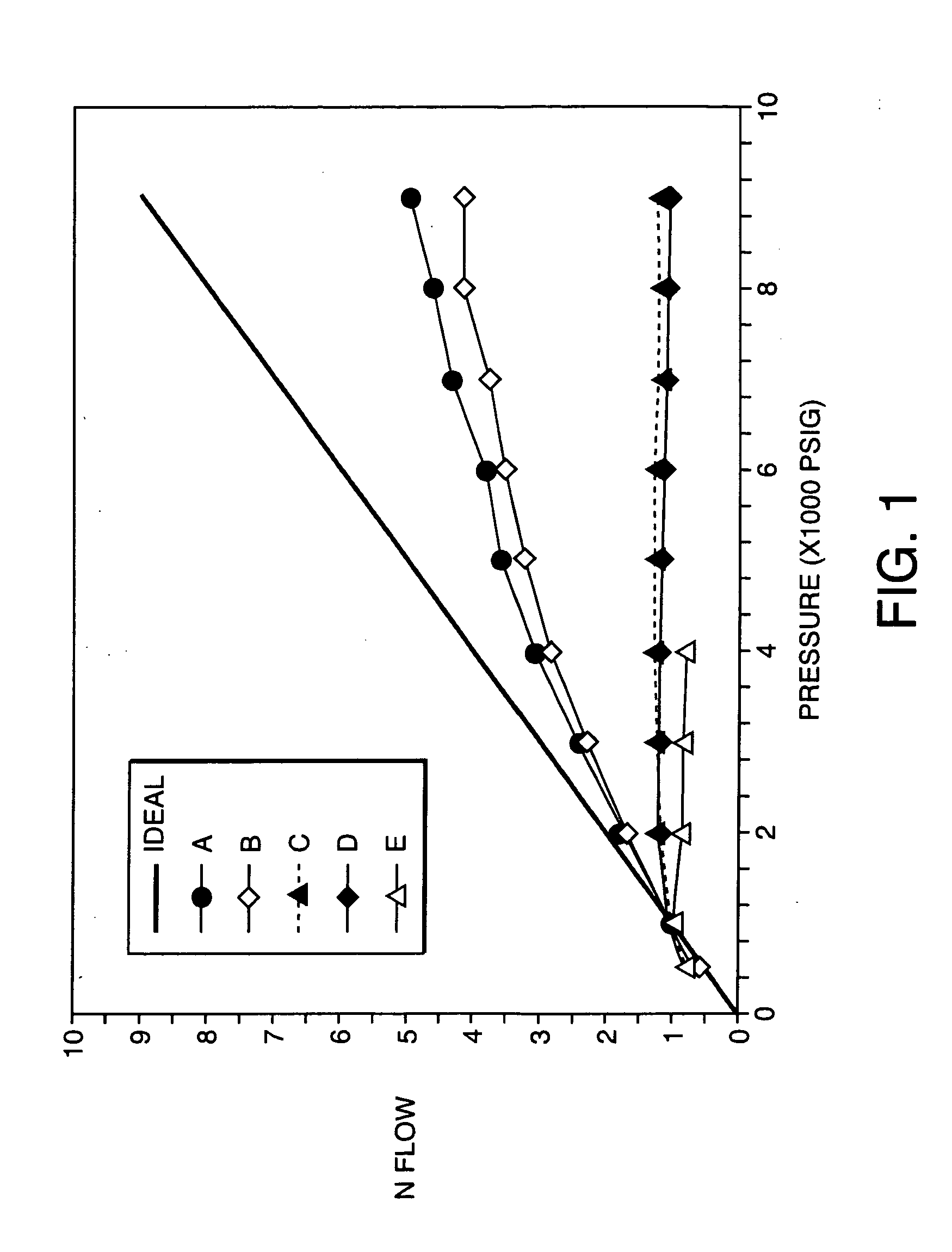
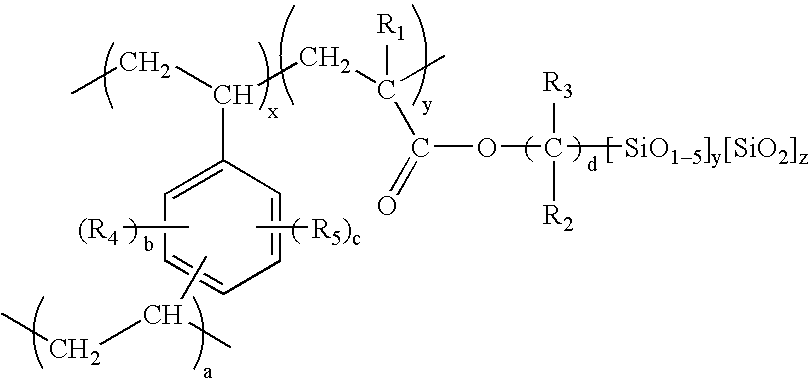
Porous inorganic/organic homogenous copolymeric hybrid materials for chromatographic separation and process for the preparation thereof
a technology of homogenous copolymer hybrid materials and chromatographic separation, which is applied in the direction of chemical/physical processes, inorganic chemistry, component separation, etc., can solve the problems of limited hydrolytic stability, subsequent collapse of chromatographic bed, and loss of analyte retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0105] One or more organoalkoxysilanes alone or in combination with a one or more alkoxysilanes (all from Gelest Inc., Tullytown, Pa.) were mixed with an alcohol (HPLC grade, J. T. Baker, Phillipsburgh, N.J.) and 0.1 N hydrochloric acid (Aldrich Chemical, Milwaukee, Wis.) in a flask. The resulting solution was agitated and refluxed for 16 hours in an atmosphere of argon or nitrogen. Alcohol was removed from the flask via distillation at atmospheric pressure. Residual alcohol and volatile species were removed by heating at 115-140° C. for 1-2 hours in a sweeping stream of argon or nitrogen or by heating at 125° C. under reduced pressure for 1-2 hours. The resulting polyorganoalkoxysiloxanes were colorless viscous liquids. The chemical formulas are listed in Table 1 for the organotrialkoxysilanes and alkoxysilanes used to make the product polyorganoalkoxysiloxanes (POS). Specific amounts are listed in Table 2 for the starting materials used to prepare these products. Example 1e was ma...
example 2
[0107] A solution of poly(vinyl alcohol) (PVA; 87%-89% hydrolyzed; Ave Mw 13,000-23,000; Aldrich Chemical, Milwaukee, Wis.) in water was prepared by mixing and heating to 80° C. for 0.5 hours. Upon cooling, the PVA solution was combined with a solution comprising divinylbenzene (DVB; 80%; Dow Chemical, Midland, Mich.), a POS selected from Example 1,2,2′-azobisisobutyronitrile (AIBN; 98%, Aldrich Chemical), and or more of the following coporogens: 2-ethylhexanoic acid (2-EHA; Aldrich Chemical), toluene (HPLC grade, J. T. Baker, Phillipsburgh, N.J.), cyclohexanol (CXL; Aldrich Chemical), 1-methyl-2-pyrrolidinone (NMP; Aldrich Chemical). The two solutions were mixed initially using a mechanical stirrer with Teflon paddle and then emulsified by passing the mixture through a static mixer for 10 minutes under an argon flow. With continuous static mixing, the emulsification was heated to 70-80° C. in a period of 30 minutes. Thereafter, the emulsion was agitated mechanically at 70-80° C. fo...
example 3
[0108] A solution of Triton® X-45 (Aq X-45; Fluka, Milwaukee, Wis.), Triton® X-100 (Aq X-100; Fluka, Milwaukee, Wis.), or Methocel E15 (M E15, Dow, Grove City, Ohio; aqueous solution prepared by preheating water to 90° C. before addition of M E15 and cooling to 25° C.) in water and or ethanol was prepared by mixing and heating to 60° C. for 0.5-1.0 hours. In a separate flask, a solution was prepared under a nitrogen purge at ambient temperature by mixing for 0.5 hours divinylbenzene (DVB; 80%; Dow Chemical, Midland, Mich.; washed 3× in 0.1 N NaOH, 3× in water, and then dried MgSO4 from Aldrich Chemical), a POS selected from Example 1, 2,2′-azobisisobutyronitrile (AIBN; 98%, Aldrich Chemical), and one or more of the following reagents: toluene (HPLC grade, J. T. Baker, Phillipsburgh, N.J.), cyclohexanol (CXL; Aldrich, Milwaukee, Wis.), dibutylphthalate (DBP; Sigma; Milwaukee, Wis.), Triton® X-45 (Oil X-45; Fluka, Milwaukee, Wis.). For example 3f, 14 g of Pluronic® F-87 (F-87; BASF; M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com