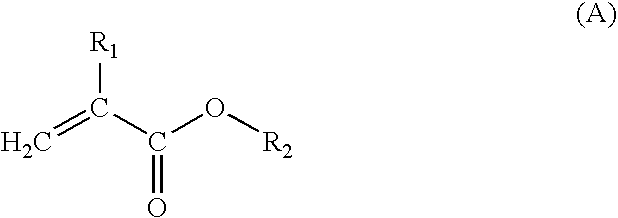
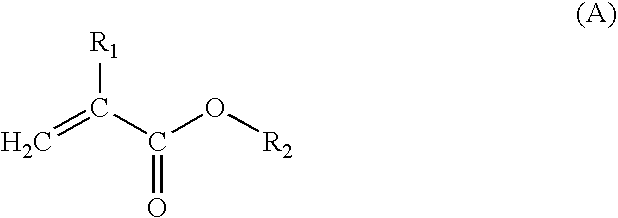
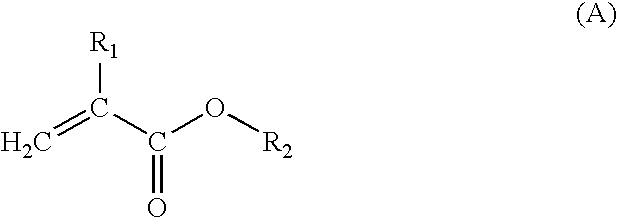
Acrylic resin composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0156] The acrylic resin solution obtained in Polymerization Example 1-1 was used as a solution of an acrylic resin (1), the acrylic resin solution obtained in Polymerization Example 2-3 was used as a solution of an acrylic resin (2), and they were mixed so that the content of non-volatile components in the acrylic resin (1) was 70 parts and the content of non-volatile components in the acrylic resin (2) was 30 parts, to obtain an ethyl acetate solution of acrylic resin composition having a non-volatile component content of 20.0%. To 100 parts of non-volatile components in the resulted solution was mixed 1.0 part of non-volatile components of a polyisocyanate-based compound (trade name: Takenate D-160N, manufactured by Mitsui-Takeda Chemical Inc.) and 0.4 part of a silane-based compound (trade name: KBM-403, manufactured by Shin-Etsu Silicones) as a cross-linking agent, to obtain an adhesive of the present invention.
Production Examples of Optical Laminated Film, and Optical Laminat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com