Supersensitive nuclear magnetic resonance imaging apparatus
a nuclear magnetic resonance imaging and supersensitive technology, applied in the direction of reradiation, measurement using nmr, instruments, etc., can solve the problems of insufficient understanding of the correlation between the function and the structure of a protein molecule at the biocellular level, the growth of a high-quality protein crystal that can be analyzed sufficiently, and the need for several months to several years to grow. , to achieve the effect of extreme performance, increased throughput and measurement sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
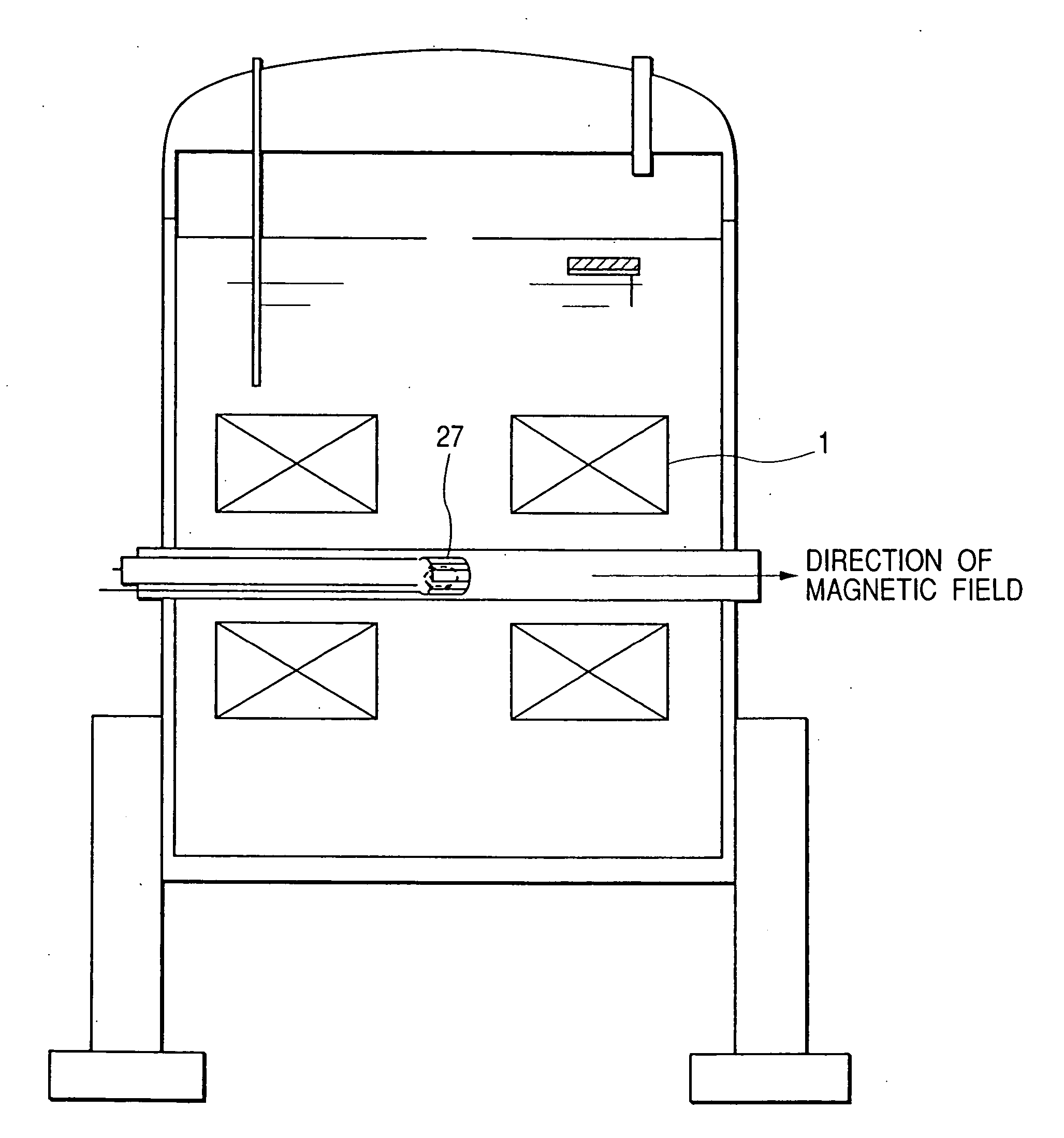
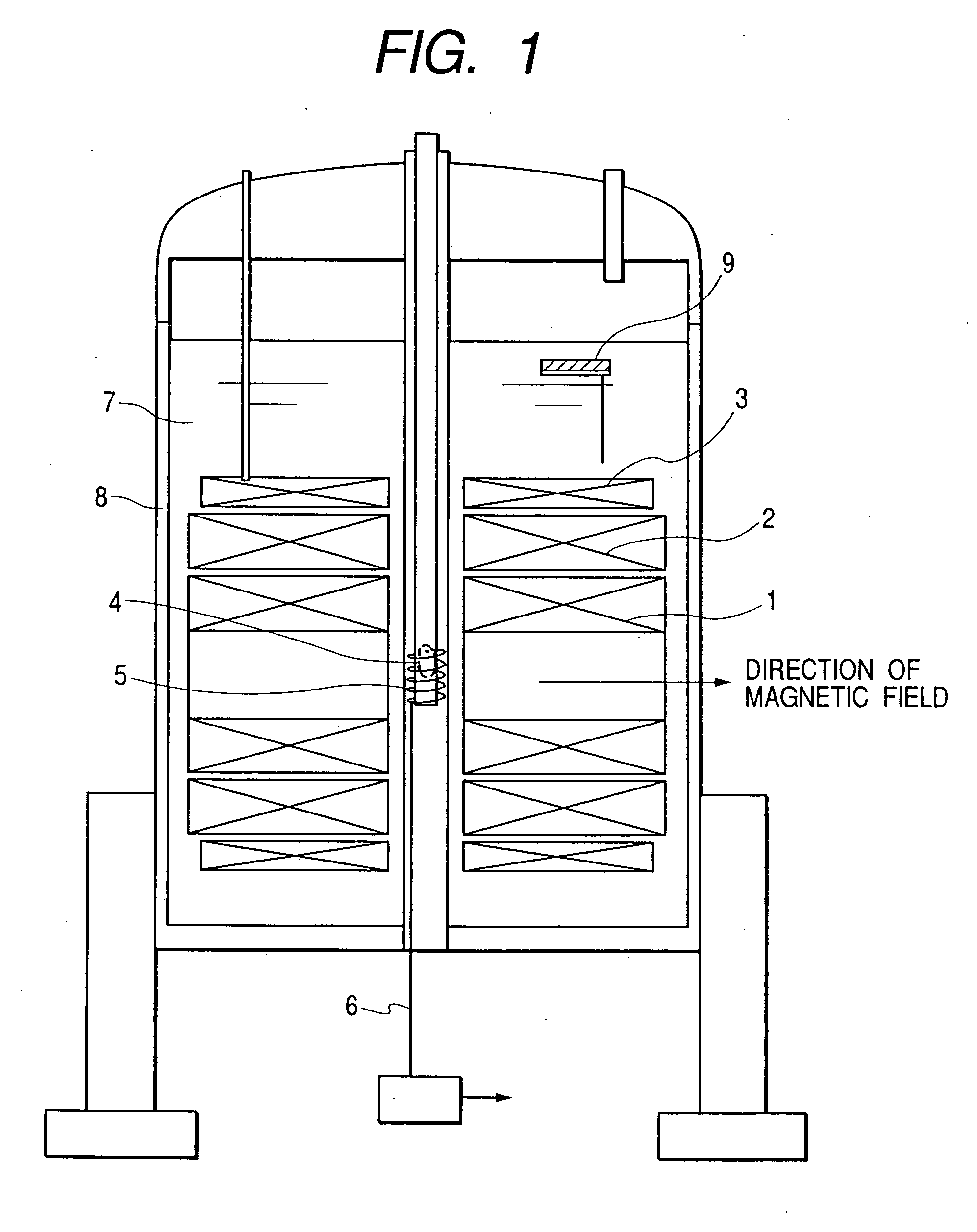
[0049] The first embodiment of the present invention is shown in FIG. 1. In the superconducting magnets 1, 2, 3, the coils are formed in such a manner that the inner side, that is the side closer to the sample, is formed of a material having a higher superconducting critical magnetic field. For example, the superconducting magnet 1 is formed of Nb3Al, the superconducting magnet 2 is formed of Nb3Sn, and the superconducting magnet 3 is formed of NbTi; and, they can be optimally combined to obtain desired values of coil-generated magnetic field and of uniformity as needed. For example, a Bi-type such as Bi2Sr2CaCu2O9 and the like, or superconducting material, such as Y1Ba2Cu3O7 and the like, may be used, or MgB2 and the like may also be used. The direction of generation of the magnetic fields of the superconducting magnet constructed of a combination thereof is horizontal.
[0050] In FIG. 1, the biosample 4, such as small animals, cells, and organic tissues, is inserted from the top of...
embodiment 2
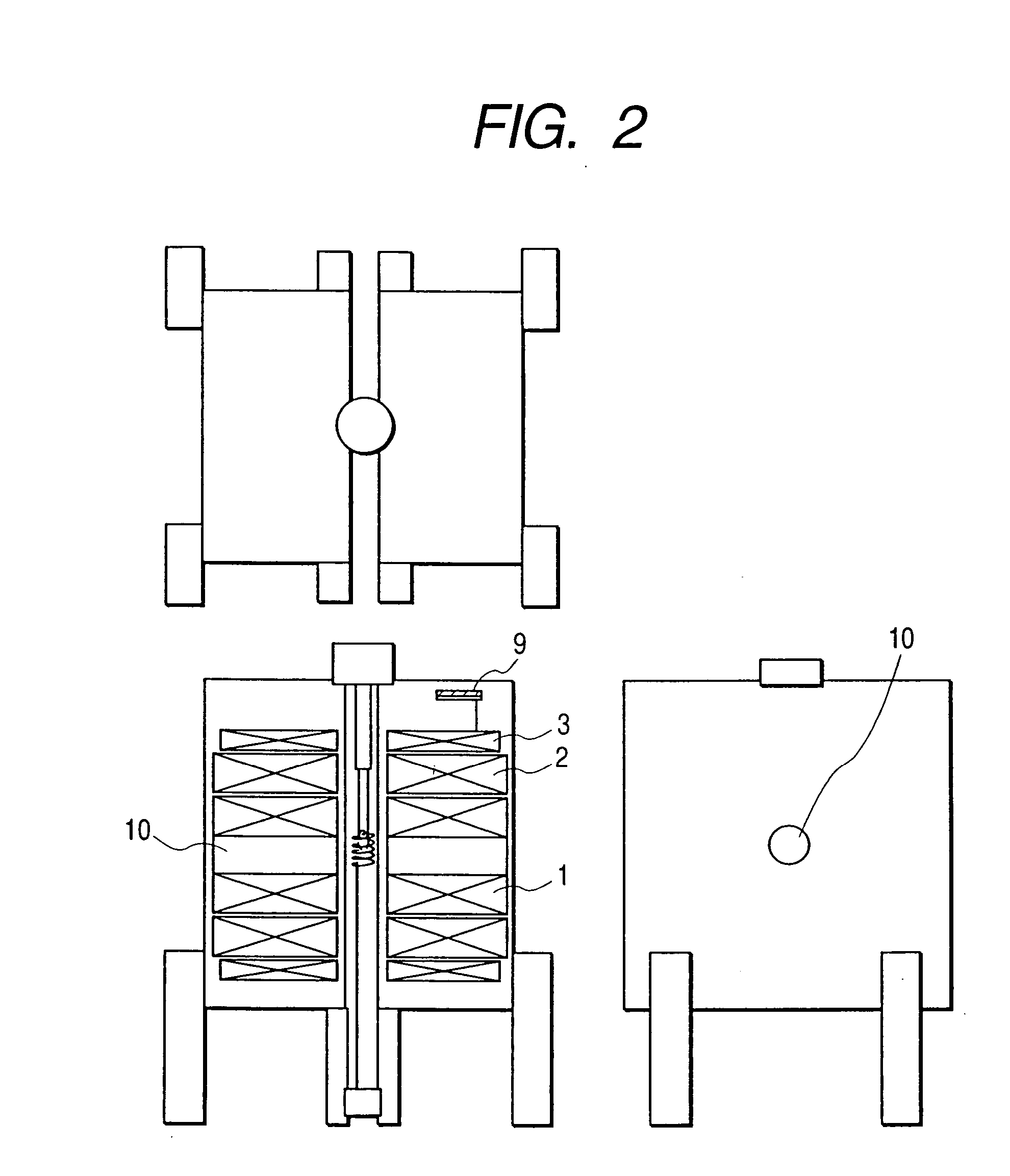
[0059] The second embodiment of the present invention is shown in FIG. 2. In this embodiment, the construction is generally identical to the first embodiment, but the low temperature container is divided by the left and right superconducting magnets to provide an openness to the available space for the user. In other words, since there is an open space 10 around the sample chamber, unlike the hermetical sample space in the previously known construction, the dynamic behavior of the liming organisms, such as photosynthesis, can be measured, while performing light irradiation or laser beam irradiation on the sample easily. Since the dynamic NMR signal can be observed in this manner, for example, the signal transmission or reaction of photosynthesis of the protein can be inspected.
[0060] When performing such special experiments, liquid helium is pumped to cool down and operate the apparatus at 1.8 K, so that the superconducting magnet can be operated approximately at 900 MHz (21.1 T) a...
embodiment 3
[0061] A third embodiment of the present invention is shown in FIG. 3. In this embodiment, the biosample is inserted into the apparatus from the top, and the measurement probe is inserted from the side. In this arrangement, the frame 11, that is provided with an anti-vibration device, can be lowered; and, thus the height of the apparatus can be lowered, whereby the operability is improved, and vibrations propagated from the floor to the apparatus can be reduced. Therefore, an economically effective system, which is superior in installability and maintainability, can be provided.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com