Protein hydrolysate rich in tripeptides
a tripeptide and protein hydrolysate technology, applied in the field of protein hydrolysate, can solve the problems of residual immunogenic materials, low yield of nutritionally indispensible amino acids, and bitter tas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Properties of the Tripeptidylpeptidase Encoded by Gene 12 (TPAP-A) of Aspergillus niger
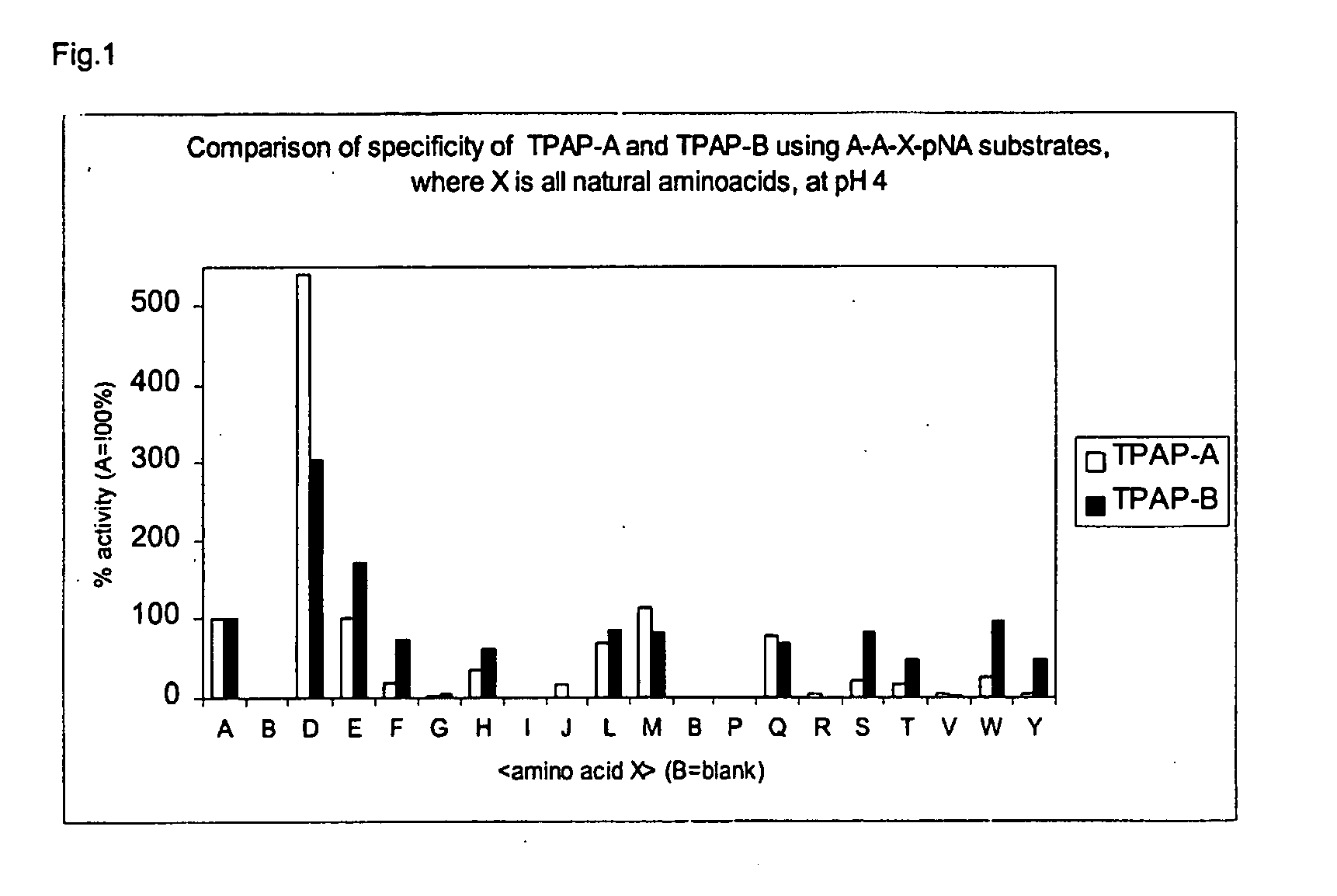
[0077] The enzyme encoded by gene 12 (described in our copending application PCT / EP02 / 01984) was overproduced in an A. niger host cell and chromatographically purified. Purification was carried out on a Resource Q column in 50 millimol / liter acetate pH 4.5. Elution by increasing the NaCl concentration yielded the enzyme in a sharp activity peak. Activity was measured by incubation with the synthetic peptide Ala-Ala-Phe-pNA. The solution with the purified enzyme contained 8 units / ml if tested on the synthetic tripeptide Ala-Ala-Phe-pNA at pH 4.0 and 60 degrees C. (see Materials & Methods section).
[0078] In a first experiment, the pure enzyme was incubated at pH 5 and 50 degrees C. with two different synthetic chromogenic substrates i.e. Ala-Ala-Phe-pNA and Ala-Phe-pNA (both from Bachem, Switserland). Stock solutions of these peptides were made in DMSO which were then diluted 100× in the desired a...
example 2
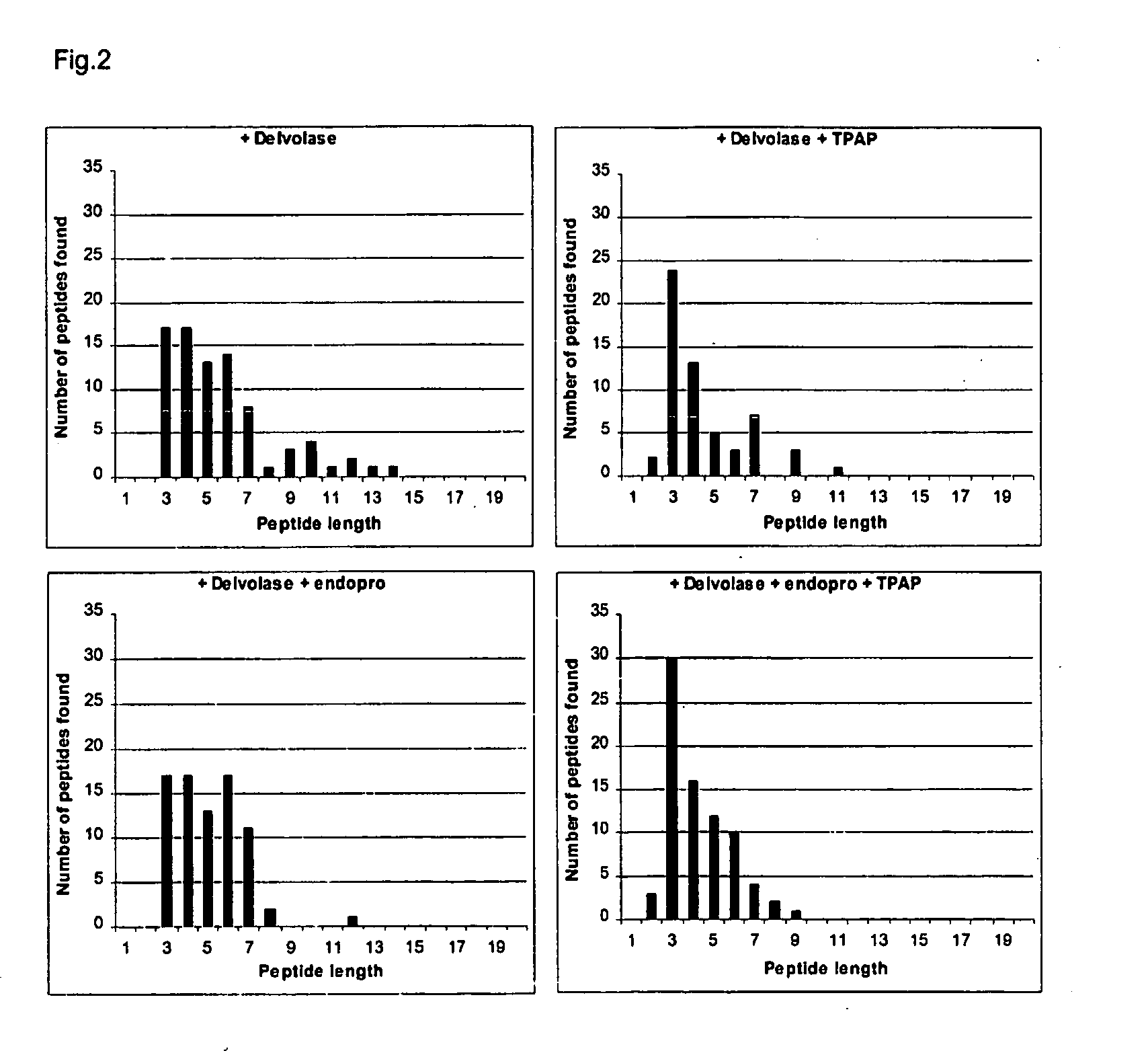
Casein hydrolysates subjected to a proline-specific endoprotease in combination with a tripeptidylaminopeptidase are non-bitter and contain a high proportion of tripeptides having carboxyterminal proline residues.
[0083] A 6% (w / w on protein) casein solution was prepared by dissolving sodium caseinate in water. After adjustment of the pH to 8.0 by NaOH, the serine protease Delvolase was added to a concentration of 4% (volume of the commercial enzyme product per weight of sodium caseinate) and the mixture was incubated for 2.5 hours at 60 degrees C. under non-pH-stat conditions. Then the reaction was stopped by lowering the pH to 5.0 using lactic acid followed by a heat treatment of 10 minutes at 90 degrees C. The solution was cooled down to 50 degrees C. and two samples were taken. The first sample (Sample A) served as a reference characterizing the material that has been subjected to the action of a broad spectrum serine protease only. The second sample was used for subsequent incu...
example 3
Frequency of Di- and Tripeptides Having Carboxyterminal Proline Residues in a Commercial Casein Based Infant Formula Product
[0088] Among the various infant formula products tested (see Example 6 in our copending application PCT / EP01 / 14480) Nutramigen (Mead Johnson, containing 14 grams of casein hydrolysate per 100 gram powder) contains the highest (i.e. 22%) molar fraction of peptides carrying C-terminal proline. In the present Example we show the results of a LC / MS analysis of this hydrolysate with a focus on its content in di and tripeptides and the frequency of such peptides having carboxyterminal proline residues.
[0089] Prior to LC / MS analysis the fatty material present in infant formulae had to be removed. As specified in the Materials & Methods section this was carried out by a hexane extraction. The aqueous phase thus obtained was centrifuged, filtered and then subjected to LC / MS analysis to characterize the various peptides present.
[0090] According to the results obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com