Balloon catheter device
a balloon and catheter technology, applied in the field of balloon catheter devices, can solve the problems of affecting the operation of the catheter, and affecting the operation of the catheter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
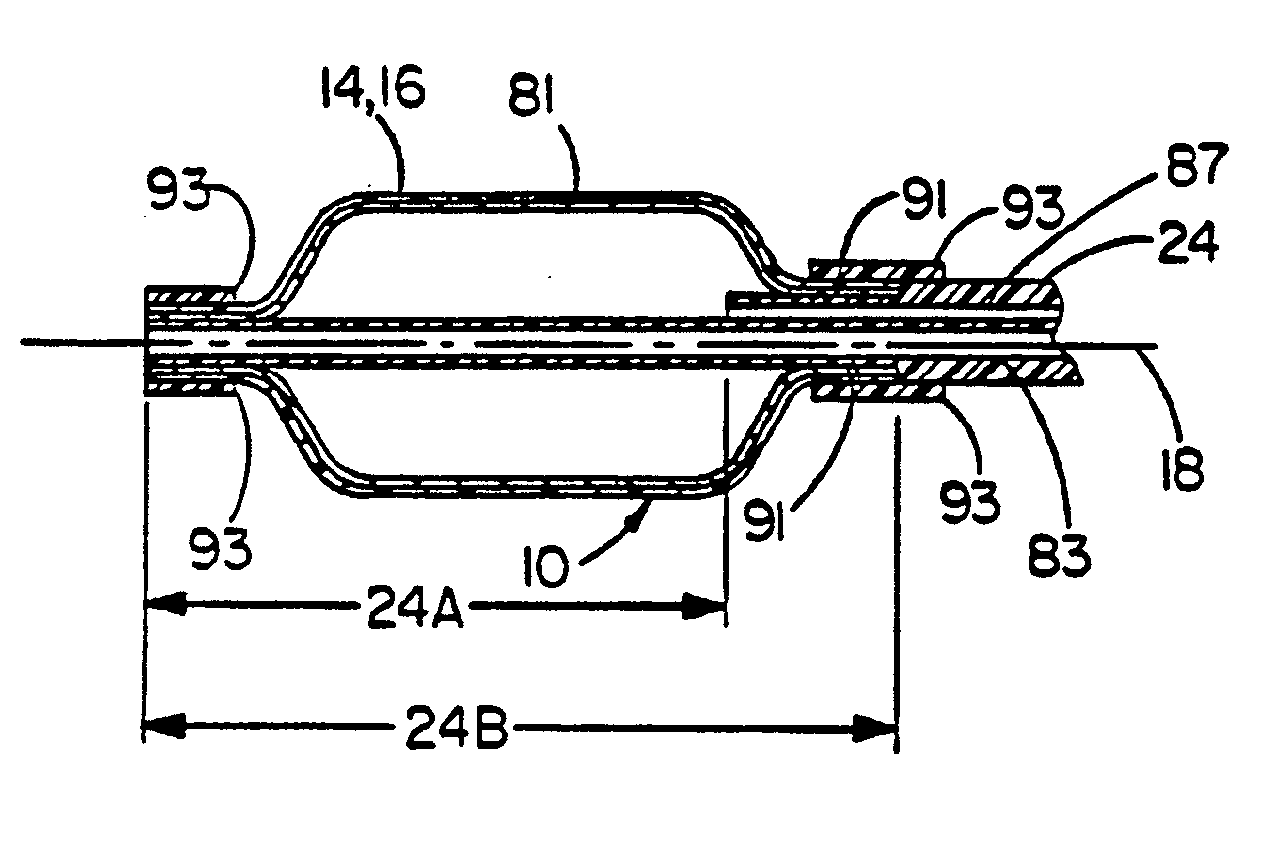
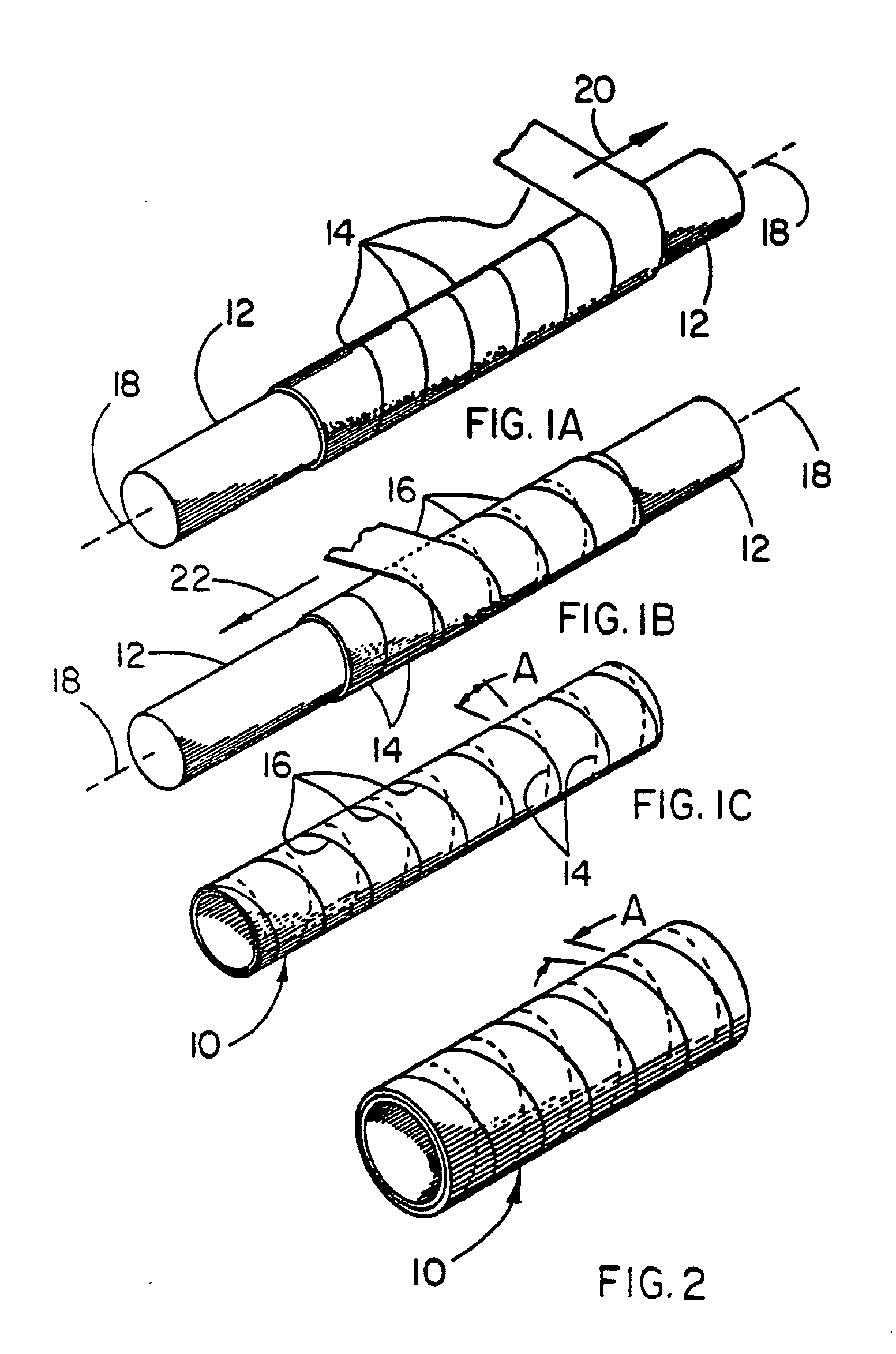
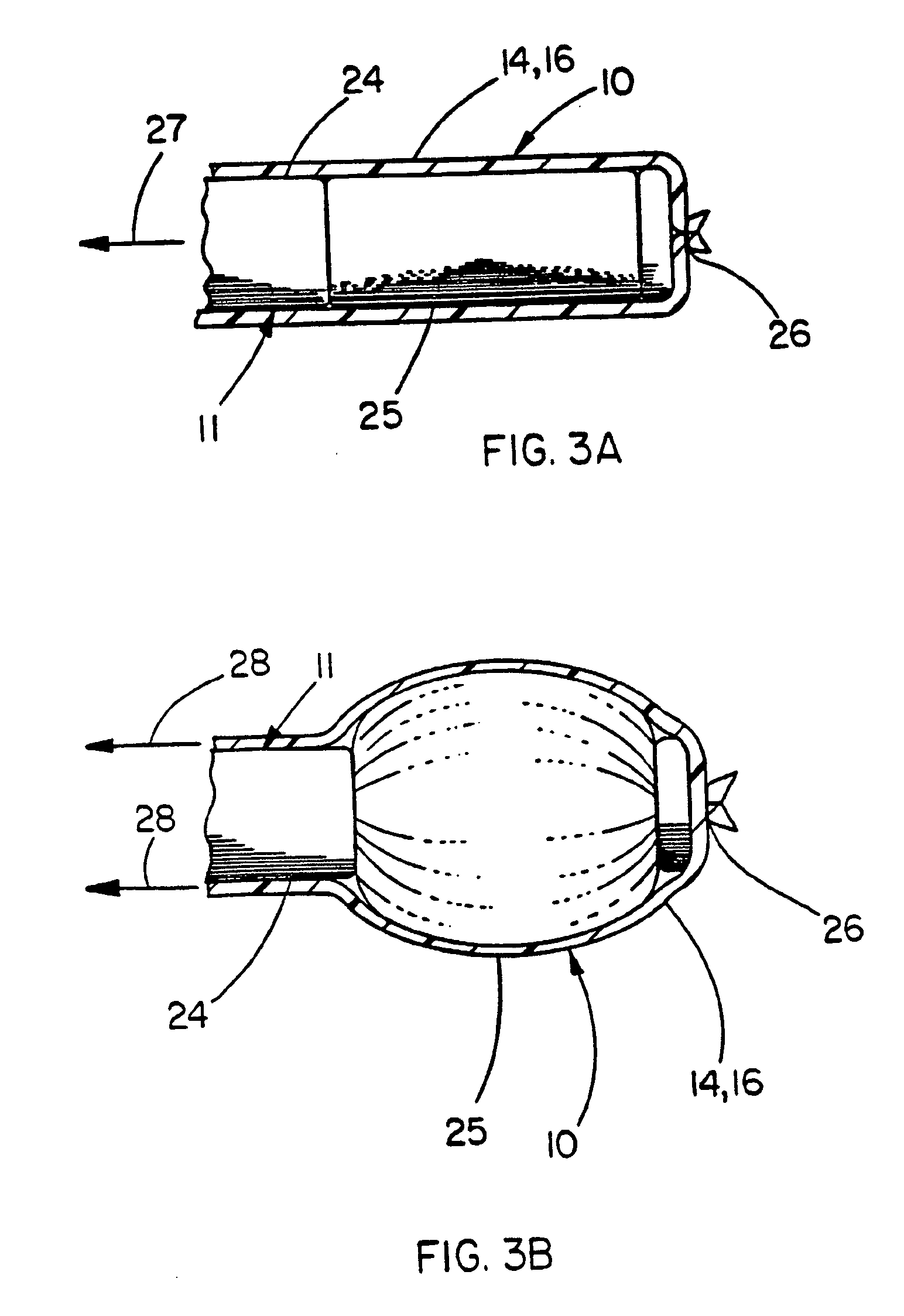
[0044] This example illustrates the use of a balloon cover of the present invention over a commercially available angioplasty balloon. The balloon cover provides a means of returning the angioplasty balloon close to its original compact geometry after inflation and subsequent deflation, as well as providing the known chemical inertness and low coefficient of friction afforded by PTFE.
[0045] The balloon used was a MATCH 35® Percutaneous Transluminal Angioplasty (PTA) Catheter model number B508-412, manufactured by SCHNEIDER (Minneapolis, Minn.). This balloon when measured immediately after being removed from the protective sheath provided by the manufacturer had a minimum dimension of 2.04 mm and a maximum dimension of 2.42 mm. These measurements were taken from approximately the center of the balloon, as defined by the midpoint between the circumferentially-oriented radiopaque marker bands located at both ends of the balloon. A Lasermike model 183, manufactured by Lasermike, (Dayto...
example 2
[0051] This example illustrates the use of a balloon cover over a commercially available latex embolectomy balloon. The balloon cover provides a defined limit to the growth of the embolectomy balloon, a substantial increase in burst strength, and the known chemical inertness and low coefficient of friction afforded by PTFE.
[0052] The balloon used was a Fogarty® Thru-Lumen Embolectomy Catheter model 12TL0805F manufactured by Baxter Healthcare Corporation (Irvine, Calif.). This natural rubber latex balloon when measured immediately after being removed from the protective sheath provided by the manufacturer had a minimum dimension of 1.98 mm and a maximum dimension of 2.02 mm. These measurements were taken from approximately the center of the balloon, as defined by the midpoint between the radiopaque marker bands. A Lasermike model 183, manufactured by Lasermike, (Dayton, Ohio) was used to make the measurements while the balloon was rotated about its longitudinal axis. The shaft onto ...
example 3
[0056] This example illustrates the use of a composite material in a balloon application. A balloon made from the composite material described below exhibits a predictable inflated diameter, high strength, exceptional compaction ratio and compaction efficiency ratio, as well as the known chemical inertness and low coefficient of friction afforded by PTFE.
[0057] A length of SILASTIC®Rx50 Silicone Tubing manufactured by Dow Corning Corporation (Midland, Mich.) having an inner diameter of 1.5 mm and an outer diameter of 2.0 mm was fitted coaxially over a 1.1 mm stainless steel mandrel and secured at both ends. The silicone tubing was coated with a thin layer of Translucent RTV 108 Silicone Rubber Adhesive Sealant manufactured by General Electric Company (Waterford, N.Y.). An 8 mm inner diameter film tube made in the same manner described in Example 1 was fitted coaxially over the stainless steel mandrel and the silicone tubing. Tension was manually applied to the ends of the film tube...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com