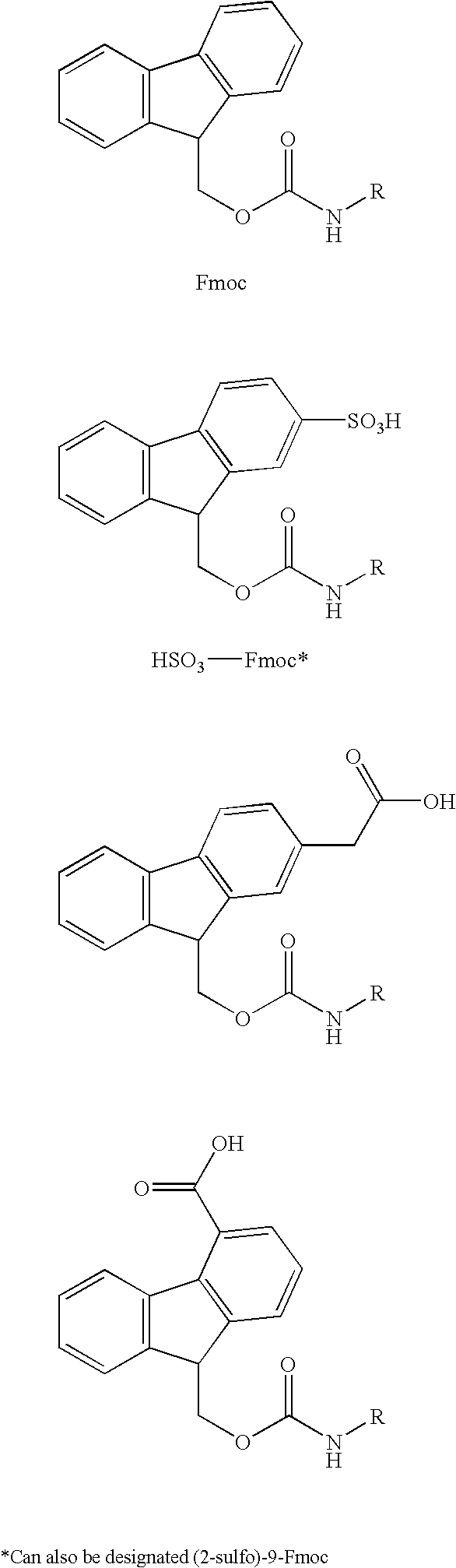
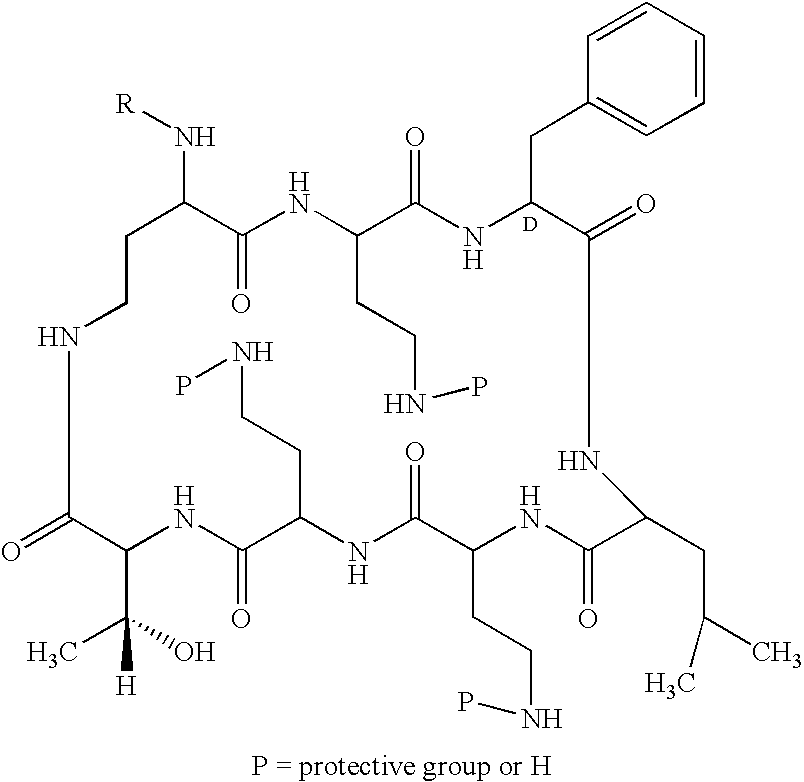
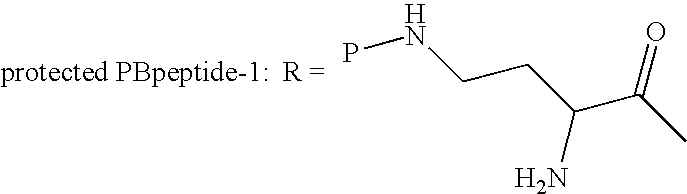
Peptide antibiotics and peptide intermediates for their prepartion
a technology of antibiotics and intermediates, applied in the field of peptide antibiotics and peptide intermediates for their preparation, can solve the problems of limited chromatographic purification methods, limited use of bacteria, and often susceptible to polymyxins, and achieve the effect of preparing for aqueous solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-[(2-Sulfo)-9-fluorenlymethoxycarbonyl)]5-polymyxin B
[0067] Polymyxin B sulfate (1.0 g., 0.841 mmol.) was dissolved in a solution of 25 ml., saturated sodium bicarbonate, 25 ml. of water and 25 ml. of tetrahydrofuran. A solution of (2-sulfo)-9-fluorenylmethoxy-N-hydroxysuccinimide (2.0 g., 4.8 mmol.) in 25 ml. of tetrahydrofuran was added in several portions over 45 min. The reaction mixture was stirred at room temperature over night and diluted with 50 ml. of water, then acidified with 25 ml of 6N hydrochloric acid to give an oily precipitate. The mixture was chilled and the aqueous layer was decanted and the oily residue was dissolved in 100 ml. of ethanol. The ethanol was evaporated under vacuum (35° C.) and the resulting solid was triturated with ethyl acetate, filtered and dried to afford 1.74 g. of product. HPLC with a gradient on a reverse phase column showed a single peak, N-[2-(sulfo)-9-fluorenlymethoxycarbonyl)]5-polymyxin B, C131H150N16O38S5, when the column eluent was ...
example 2
Preparation of the Deacylase
[0069] The deacylase is produced by culturing Actinoplanes utahensis NRRL 12052 under submerged aerobic fermentation conditions. Because single-colony isolates from a lyophile of the culture were heterogeneous for both morphology and enzyme production capability, selections were made to recover a stable, high-producing variant. Initially, multiple fermentations were carried out using inocula prepared from strain 12052. Vegetative growth from the flask yielding the best deacylating activity was plated on a differential agar (CM). Colonies were then selected for further evaluation. Generally, small colonies were better enzyme producers than the large colony types. Isolate No. 18 was selected as a small colony type and shown to be the best deacylase producer of all colonies selected. This isolate was routinely used for the production of the deacylase enzyme. CM agar contained corn steep liquor 0.5%, Bacto peptone 0.5%, soluble starch 1.0%, NaCl 0.05%, CaCl2...
example 3
Deacylation of the Protected Polymyxin B by the Enzyme in Cells
[0071] Precipitation Method: Cells from 450 ml deacylase enzyme were washed 3× with water, then brought back to original volume with 0.02 M ammonium phosphate buffer and adjusted to pH 8.0. N-[(2-Sulfo)-9-fluorenlymethoxycarbonyl)]5-polymyxin B, 897 mg, was added and the mixture was placed on a shaker at 174 rpm and maintained at 30° C. After five hours the mixture was separated by centrifuging. The clear decant was adjusted to pH 2.3 with 1 N HCl to induce precipitation and allowed to stand at room temperature. The precipitate was separated from the mixture, slurried in 80 ml water, adjusted to pH 6.5 to obtain a clear solution, and freeze-dried to obtain 400 mg of tan powder, the semi-purified salt of the protected peptide, N-[(2-Sulfo)-9-fluorenlymethoxycarbonyl]5-polymyxin peptide [(NaSO3-Fmoc)5-PBP-3]. The cells were extracted with methanol / water to obtain additional material.
[0072] Resin Method: Cells from 1 lite...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com