Atomic layer deposition of noble metals
a noble metal and atomic layer technology, applied in the field of atomic layer deposition of noble metals, can solve the problems of inability to produce many useful cvd precursors, high cost of palladium, and inability to meet the requirements of atomic layer deposition,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
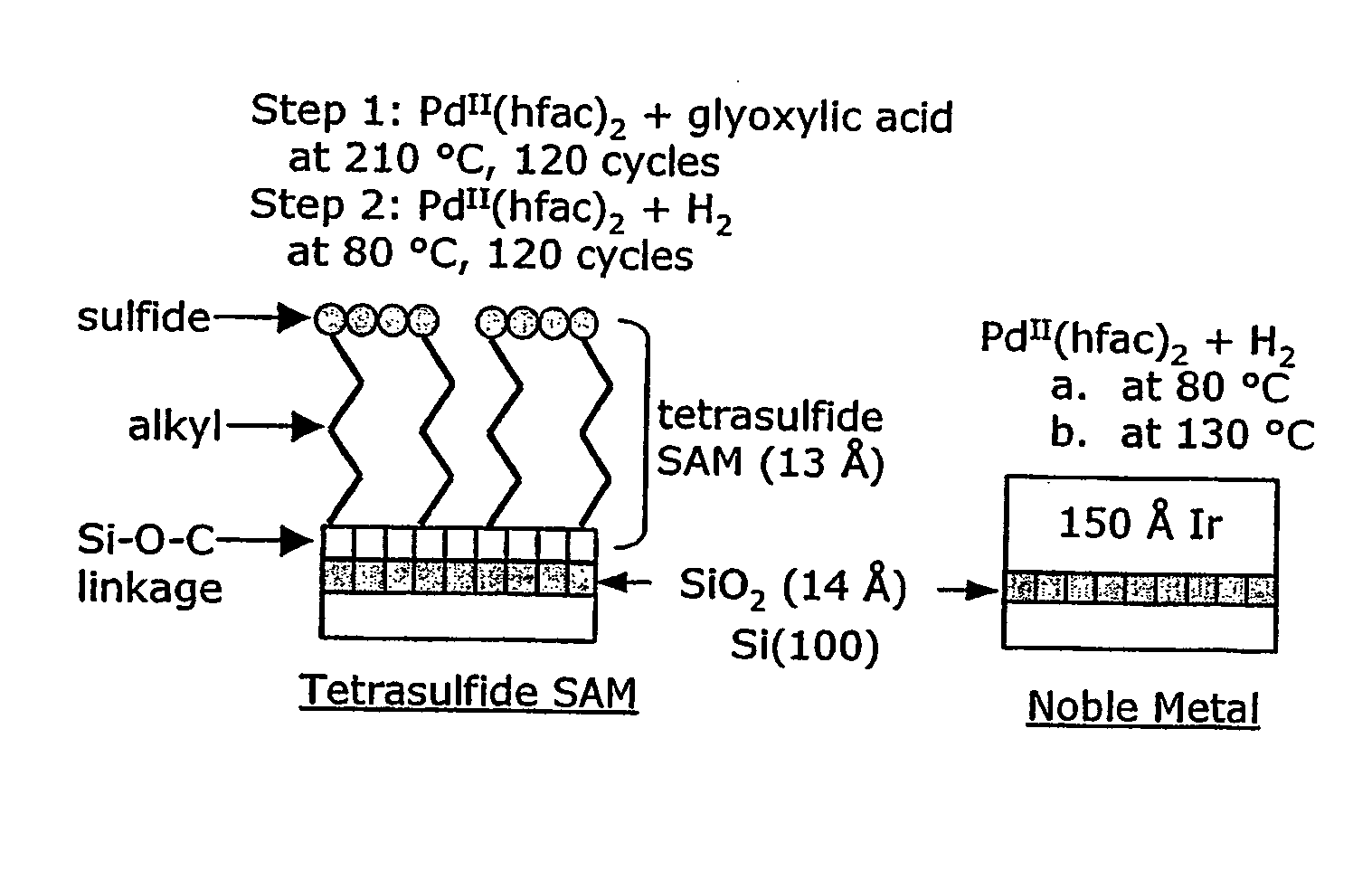
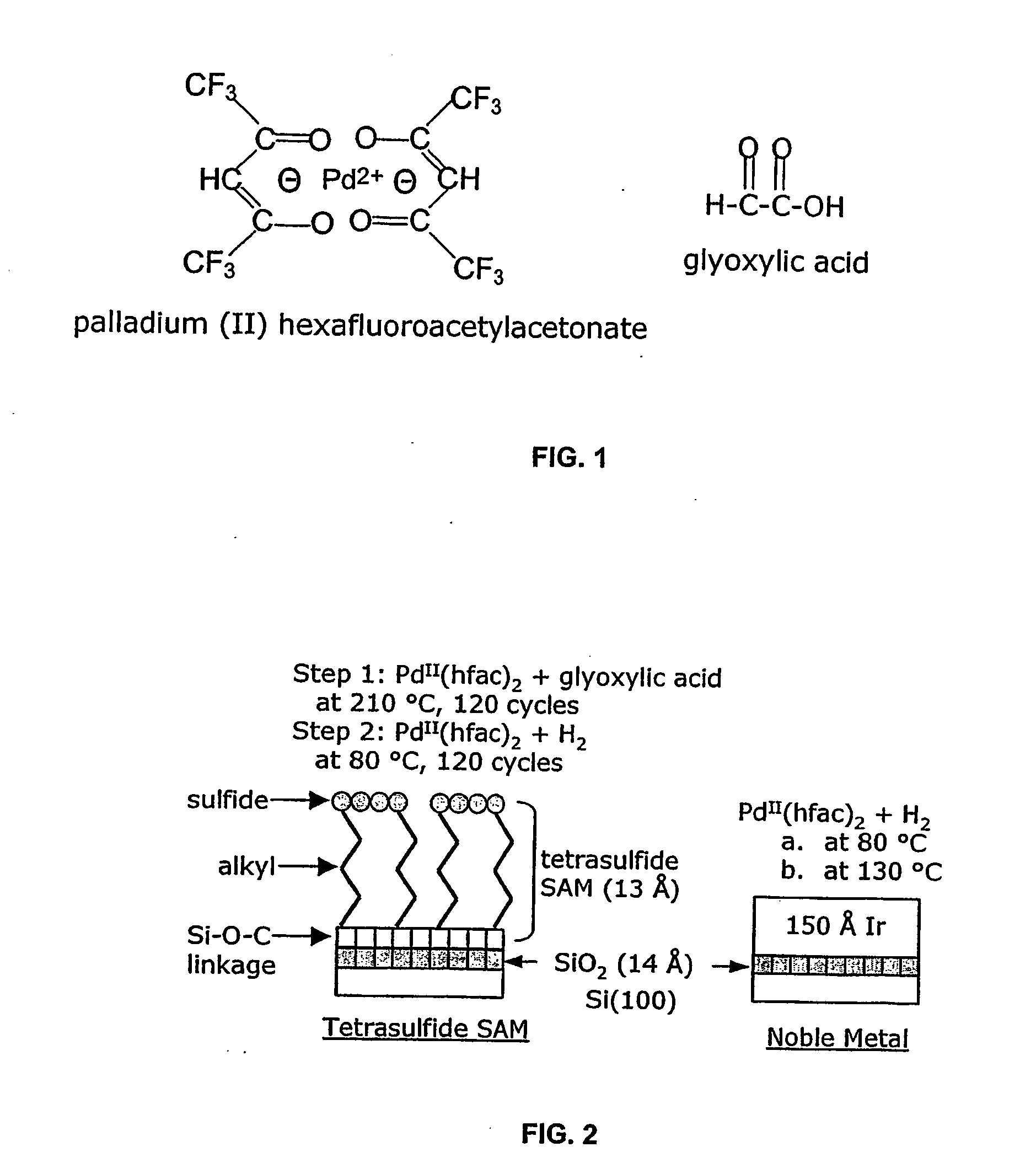
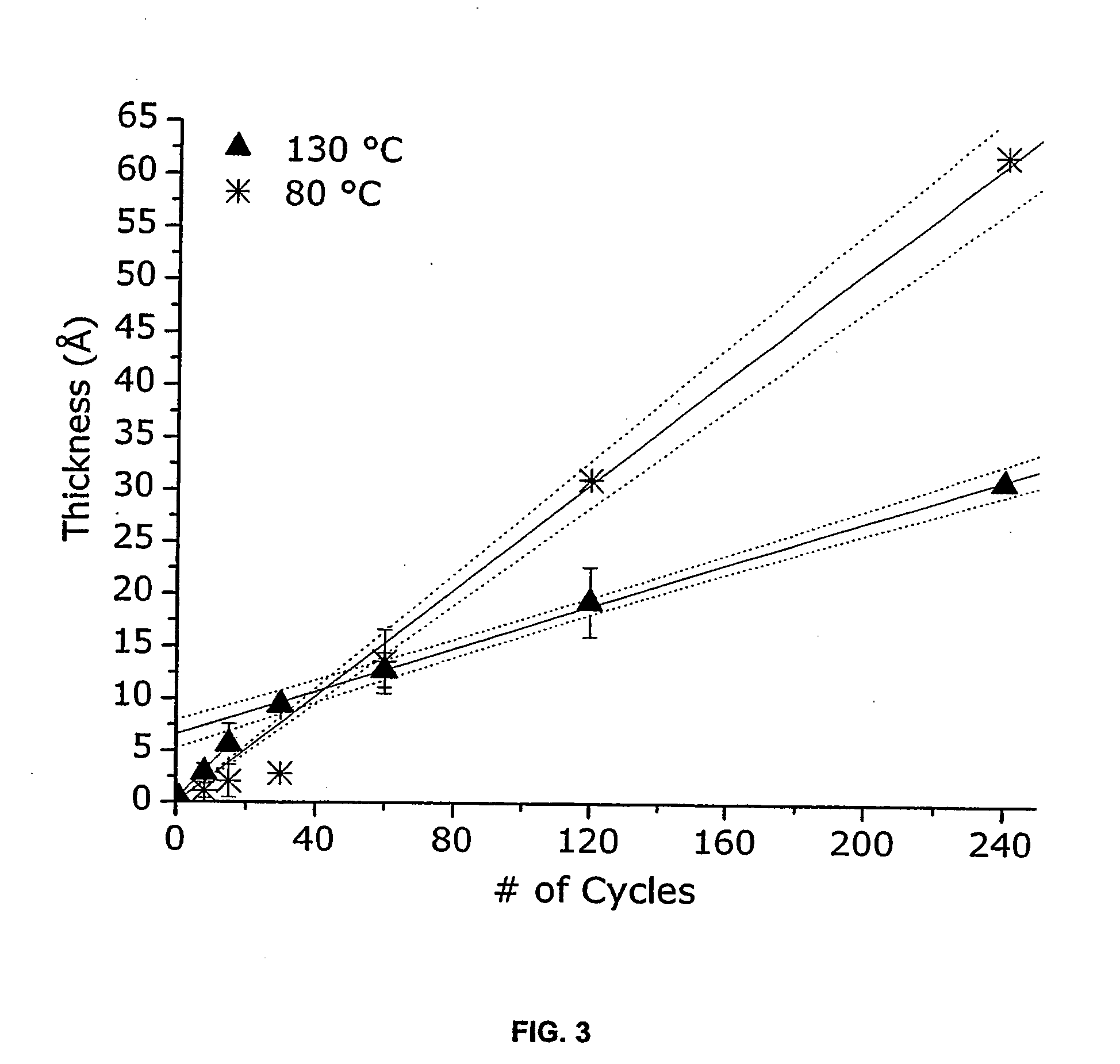
[0022] Metals that may be deposited by the ALD processes of the present invention include Pd, Pt, Ru, Rh and Ir, and particularly Pd. Suitable precursors for the metals sublime without decomposition, exhibit self-limiting chemistry and are stable at deposition temperatures. Further, they should be susceptible to decomposition on the substrate and fragments of the ligand(s) should be removable there from. Precursors are typically coordination compounds based on bidentate ligands such as β-diketonates or carboxylates. The β-diketonates are particularly useful. Examples of β-diketonate ligands include hfac, acetylacetonate (acac), tfac, fod, and tetramethylheptanedionate (tmhd). Adducts of the coordination compounds with Lewis bases may be also be utilized. Examples of Lewis bases that may be suitable include pyridine, 4,4′ bipyridyl, imidazole, ammonia, n-propanol, n-butanol, and methanol. Examples of coordination compounds that are typically suitable as precursors include Pd(hfac)2, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com