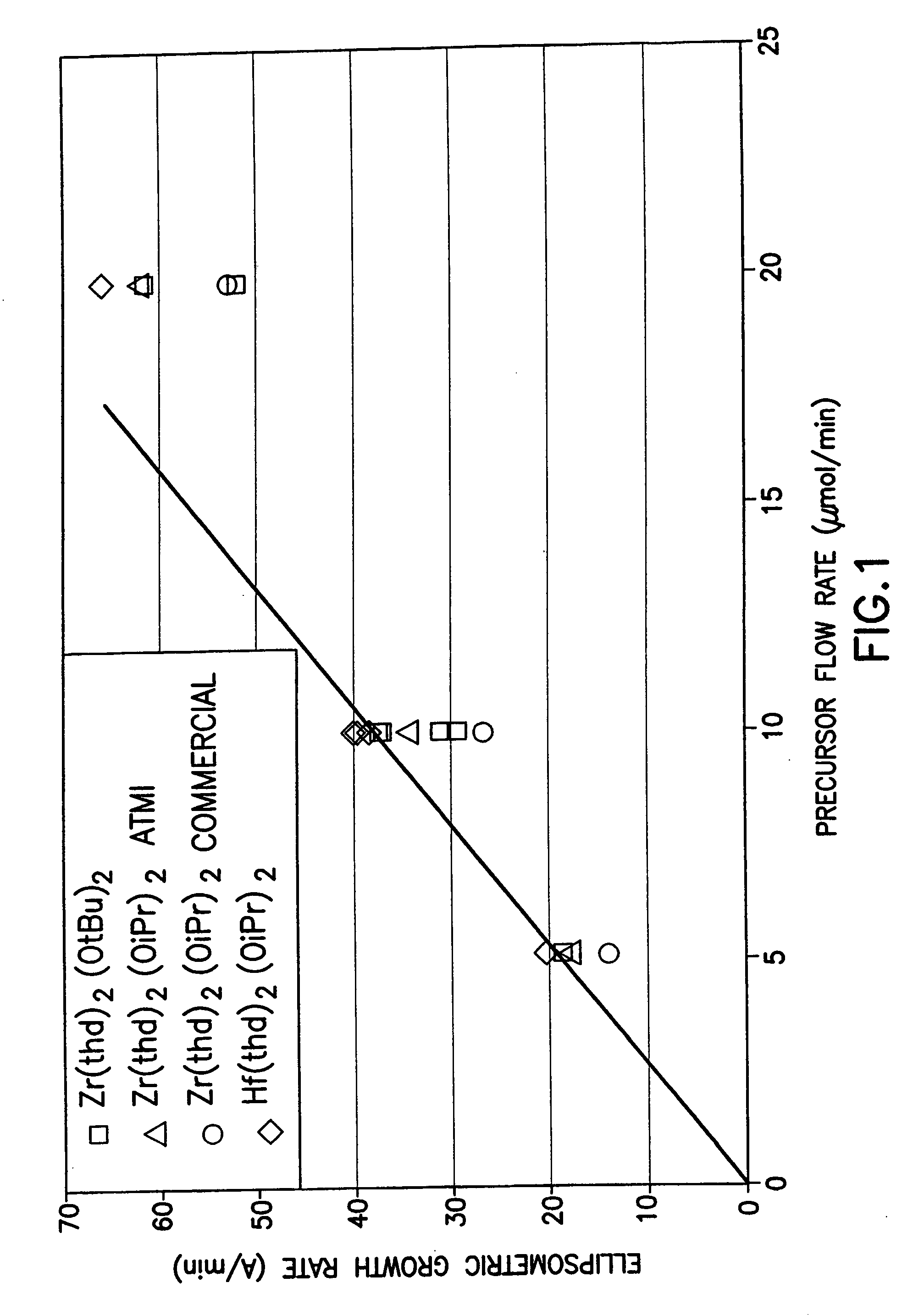
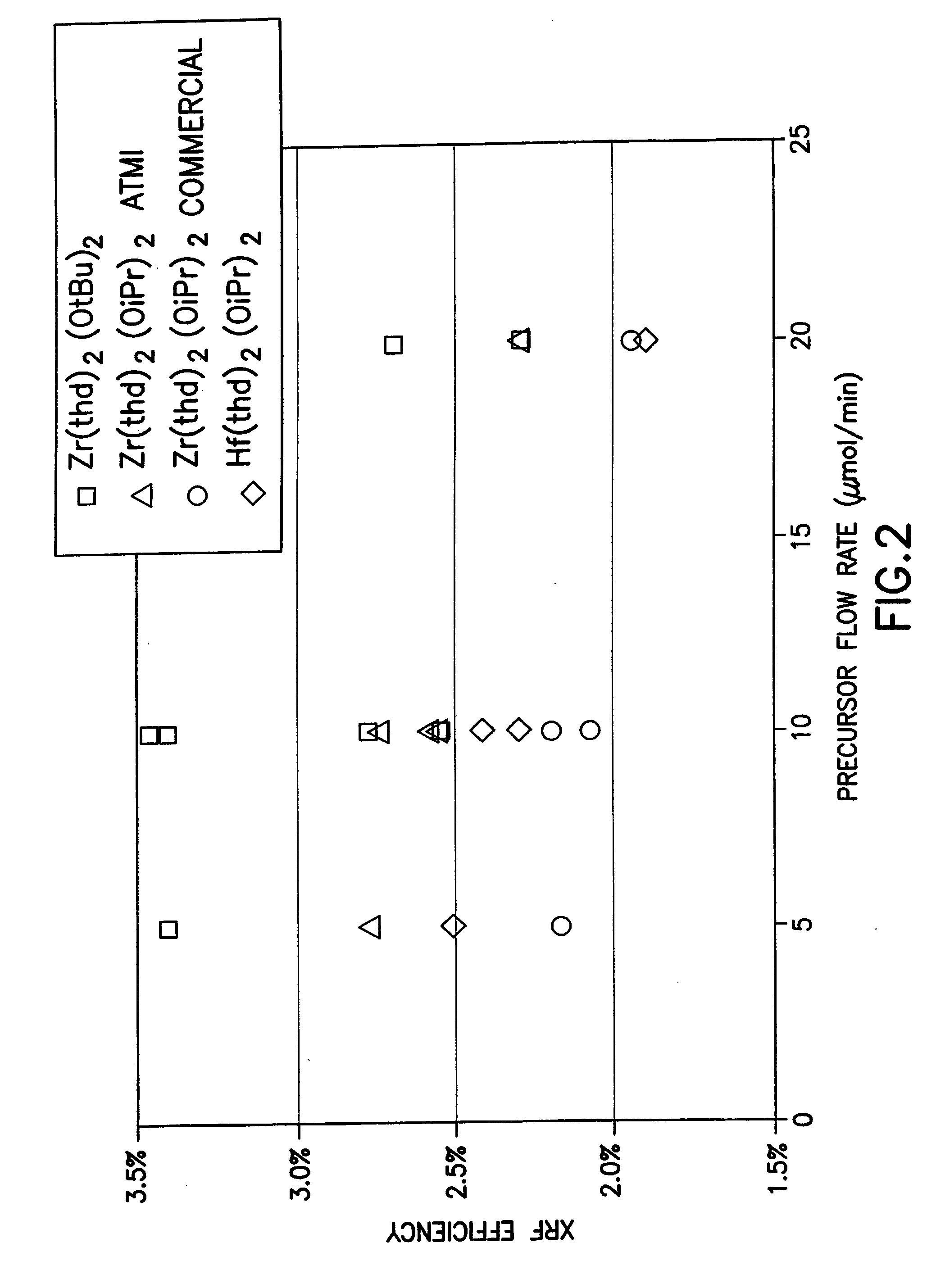
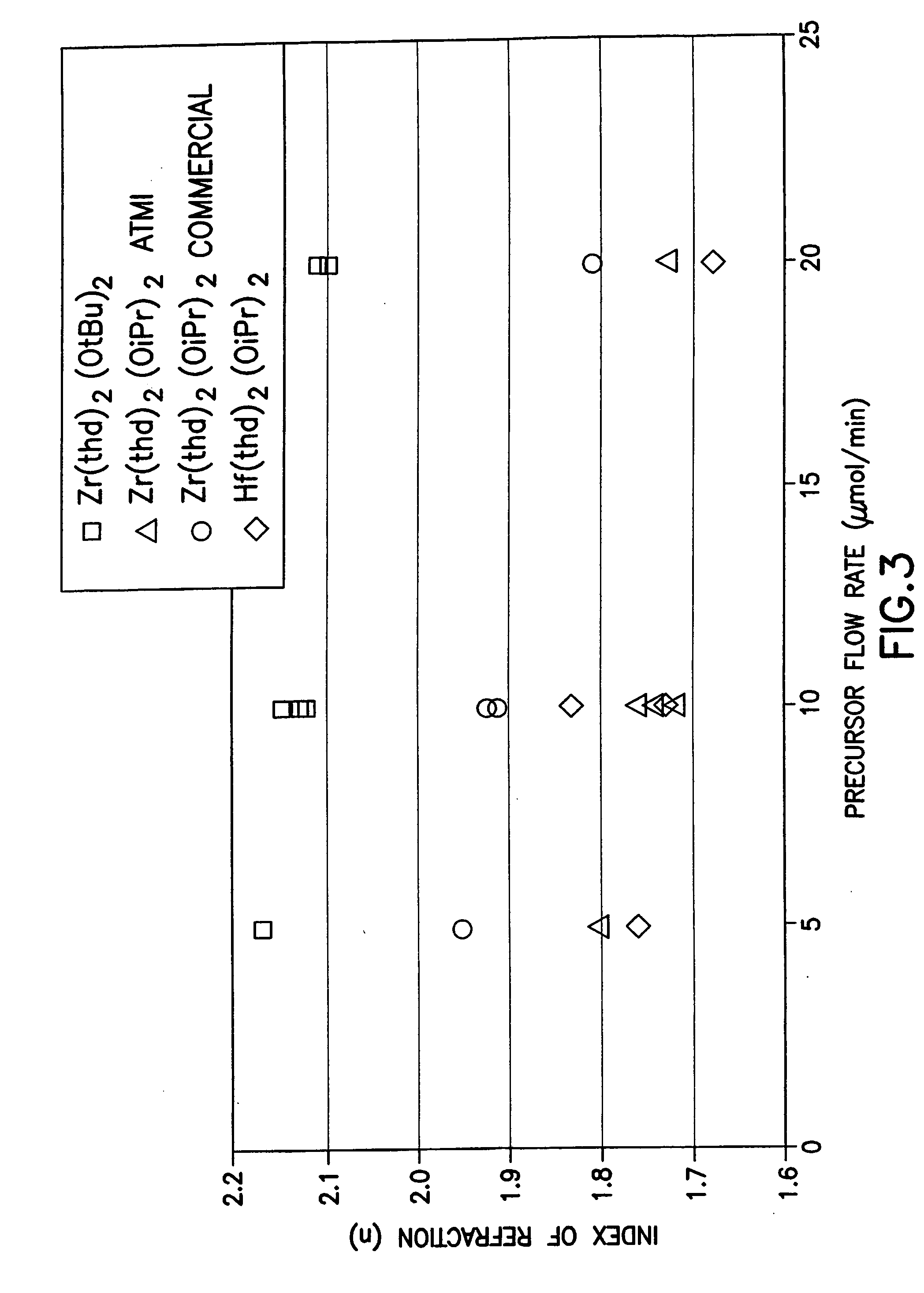
Source reagent compositions for CVD formation of high dielectric constant and ferroelectric metal oxide thin films and method of using same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
NMR Characterization of Cis- and Trans-Equilibration of Zr(thd)2(O-iPr)2
[0112] A sample of Zr(thd)2(O-iPr)2 is dissolved in deuterated benzene solvent. FIGS. 4a-4c, show the 1H NMR (C6D6), δ(ppm), spectra of a single sample of Zr(thd)2(O-iPr)2 over a period of approximately fourteen days. The original sample in FIG. 4a shows the majority of the compound to be in the cis-phase 1.15 (s, 36H=4x-C(CH3)3 of thd ligands) and 5.92 (s, 2H=2xCH of thd ligands) with a detectable amount of the trans-isomer at 1.24 (s, 36H=4xC(CH3)3 of thd ligands) and 5.97 (s, 2H=2xCH of thd ligands). FIGS. 4b-4c further evidence the cis- to trans-equilibration of the sample over time as the peaks at 1.24(s, 36H=4x-C(CH3)3 of thd ligands) and 5.97 (s, 2H=2xCH of thd ligands) increase over the fourteen-day period (FIG. 4b time lapse=4 days, FIG. 4c time lapse=14 days). Such isomerization is accompanied by a disproportionation / dimerization reaction, leading to a less volatile, less soluble dinuclear species of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com