Compact apparatus for noninvasive measurement of glucose through near-infrared spectroscopy
a near-infrared spectroscopy and compact technology, applied in the direction of spectroscopy diagnostics, instruments, applications, etc., can solve the problems of inability to accurately measure the glucose content of the glucose sample, the current monitoring technique discourages regular use, and the production and use of insulin are improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
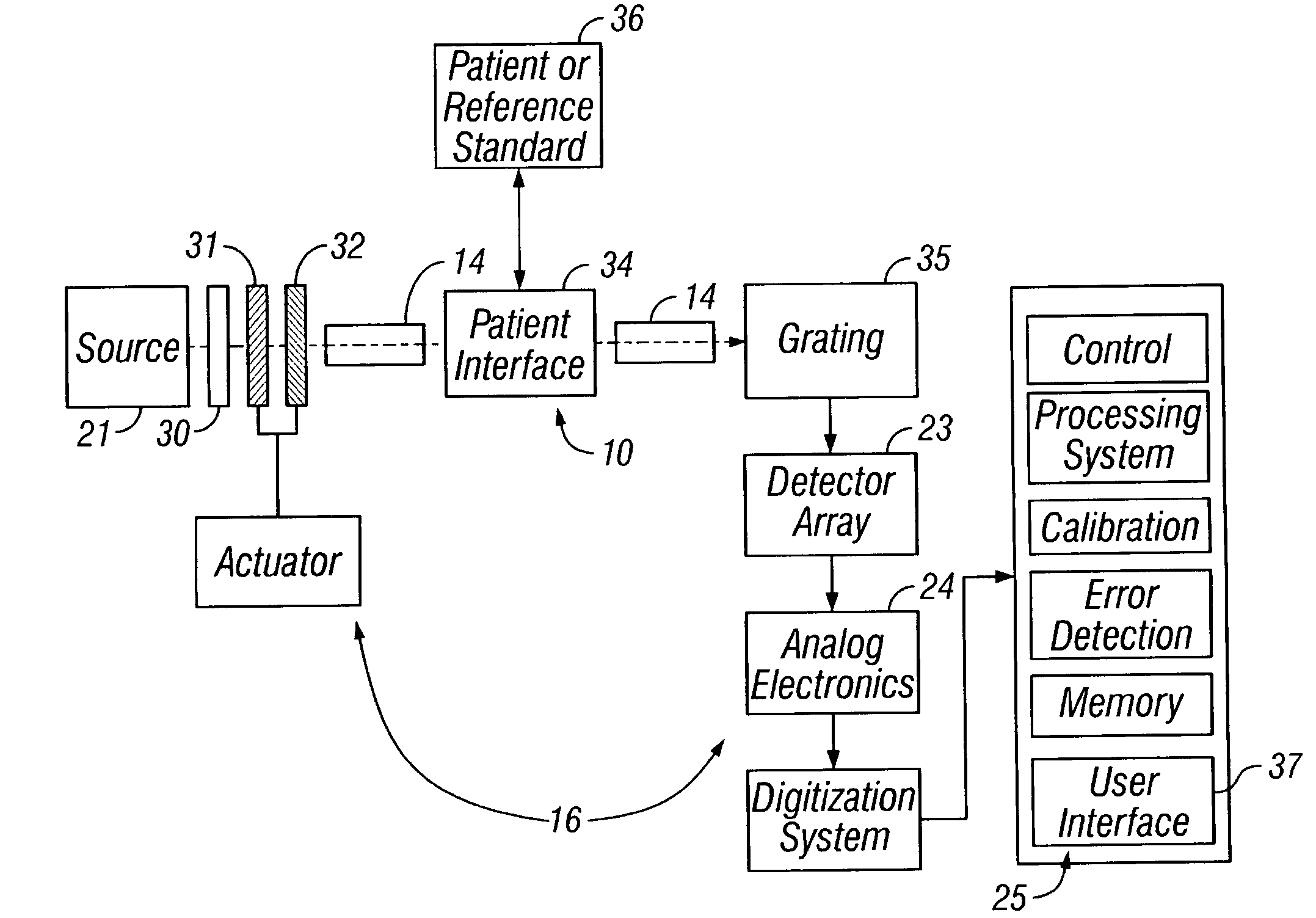
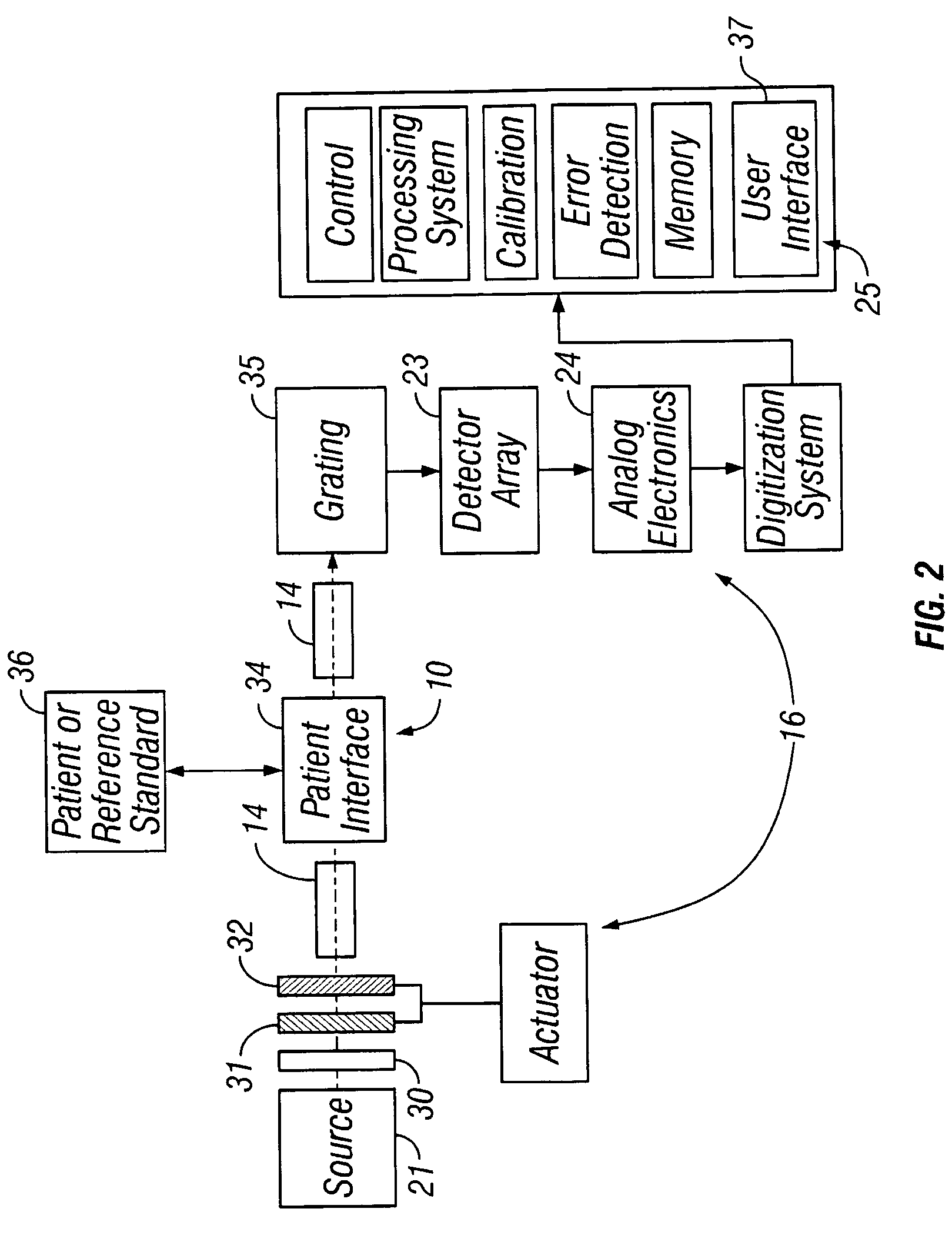
[0145] A number of instrument configurations of the alternative embodiment are presented below. Those skilled in the art will recognize that permutations and combinations of these embodiments are possible.
[0146] In the simplest embodiment, the LEDs may illuminate the sample directly, as in FIG. 8. In FIG. 8, a coupling fluid 84, as disclosed above, is shown provides between the device and the tissue sample. An optional mixing chamber with a reflective surface may be used between the LEDs 80 and the optical window 81 to provide a nearly uniform distribution onto the tissue region 82 surrounding the detection fiber 83. A spacer 85 may also be provided between the fiber and the LEDs. In this embodiment, the LEDs are designed with a bandwidth enabling the measurement, and the LEDs are arranged in a manner that allows the sampling and detection of a particular tissue volume at a particular band of wavelengths. Each LED may be recessed into a material 91 having a reflective surface 90 as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength range | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com