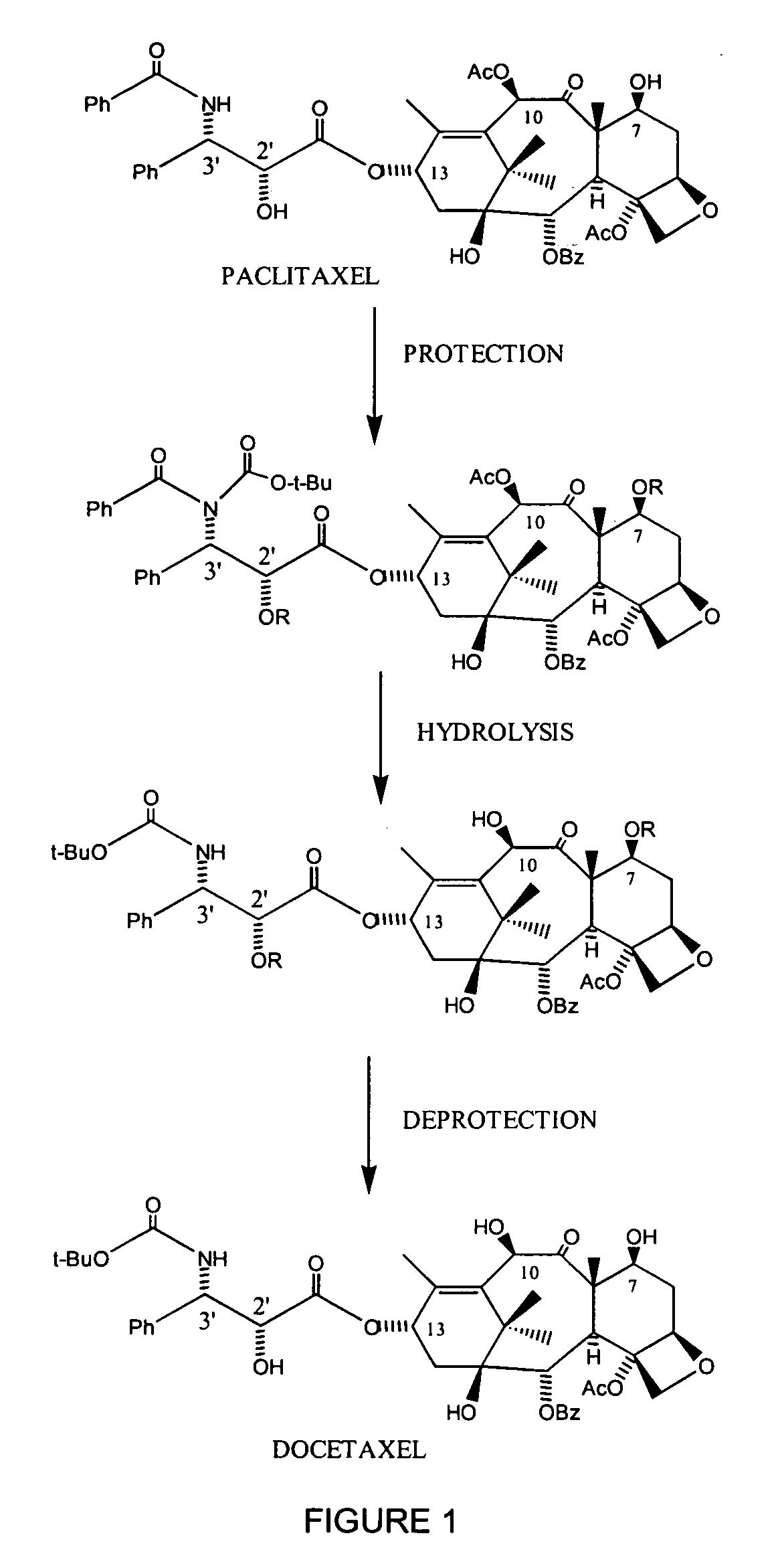
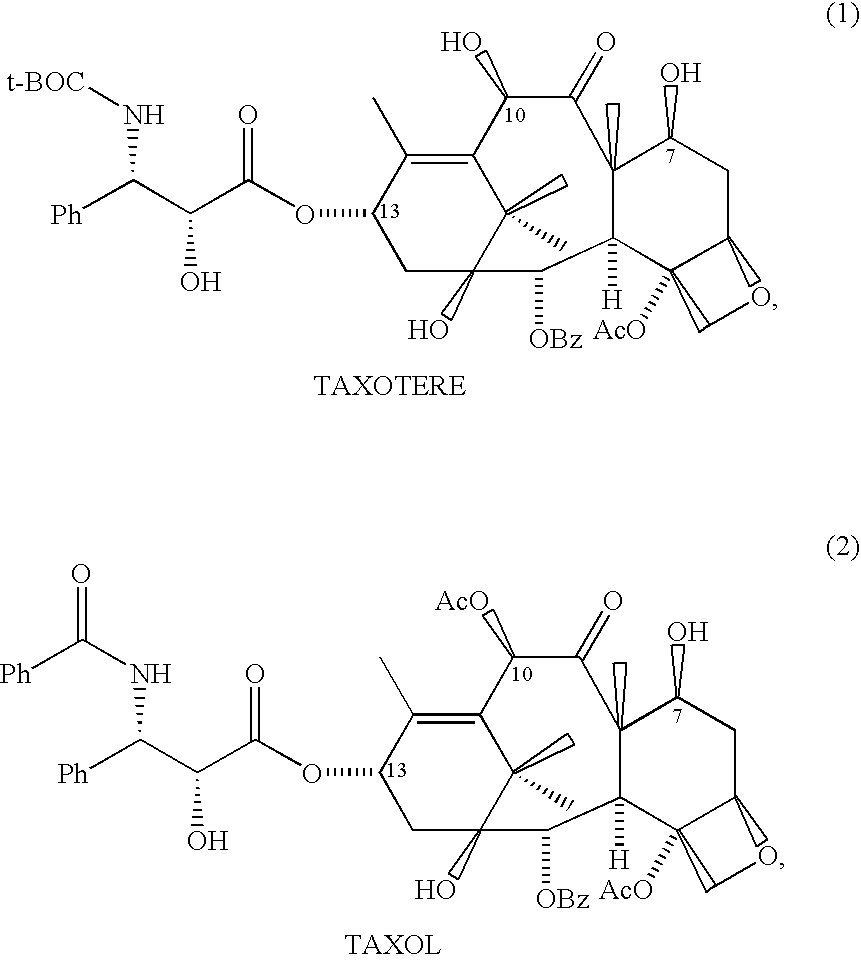
Semi-synthetic conversion of paclitaxel to docetaxel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protection of C-7, C-2′ and / or C-10 Hydroxy Groups in a One Pot Reaction
[0135] As shown in FIG. 1, to a stirred solution of paclitaxel or paclitaxel containing material, in an organic solvent, such as THF, at around low to room temperature under an argon atmosphere was treated with a hydroxy-protecting agent, such as Boc2O, dichloroacetyl chloride, acetyl chloride, TESCl or like reagents in the presence of a base, such as 4-(N,N-dimethylamino)pyridine or n-BuLi or a mixture of n-BuLi / Li-t-OBu or like bases. The reaction was stirred at this temperature for a period between 30 minutes to 2 hours until complete consumption of the starting materials, as evidenced by TLC.
[0136] Alternatively, to a stirred solution of paclitaxel or paclitaxel containing material, in an organic solvent, such as THF, at around low to room temperature under an argon atmosphere can be treated with a hydroxy-protecting agent such as ethyl vinyl ether, in the presence of a catalytic amount of p-toluenesulfoni...
example 2
Synthesis of Docetaxel
[0141] As further shown in FIG. 1, C-2′, C-7 and C-10 protected docetaxel was hydrolyzed using formic acid to remove the C-7 and / or C-10 t-Boc protecting group and then with a mixture of NaHCO3 / Na2CO3 / H2O2 to deprotect the C-2′ and / or C-10 acetate groups to yield docetaxel. In the event that the C-2′ protecting group is ethoxyethyl, the deprotection is carried out under acidic condition, such as in the presence of acetic acid. Detailed description of deprotection at the C-2′, C-7 and C-10 positions are described in U.S. patent application Ser. No. 10 / 790,622, which application is assigned to the assignee of the present invention and is incorporated herein by reference in its entirety.
example 3
Synthesis of the Primary Amine Derivative of Paclitaxel
[0142] To a solution of paclitaxel (0.76 mmol) or a paclitaxel containing material in glacial acetic acid (2.5 ml) and acetic anhydride (5 ml) at 0° C. is added NaNO2 (7.6 mmol). The resulting solution can be stirred under argon at 0° C. for 16 h and then poured over ice and extracted with diethyl ether. The combined organic extracts can be washed with water, 5% Na2CO3, water and saturated NaCl and dried over MgSO4. The dry extracts can be filtered and then concentrated in vacuo, and the crude product is purified by column chromatography using mixtures of hexane-ethyl acetate to afford the pure product.
[0143] To the above solution in tetrahydrofuran is added a 1.0 N solution of lithium hydroxide. The solution was stirred for 12 h at room temperature. After the removal of tetrahydrofuran in vacuo, the basic aqueous residue can be acidified by the addition of 10% acetic acid and extracted with ether. Dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com