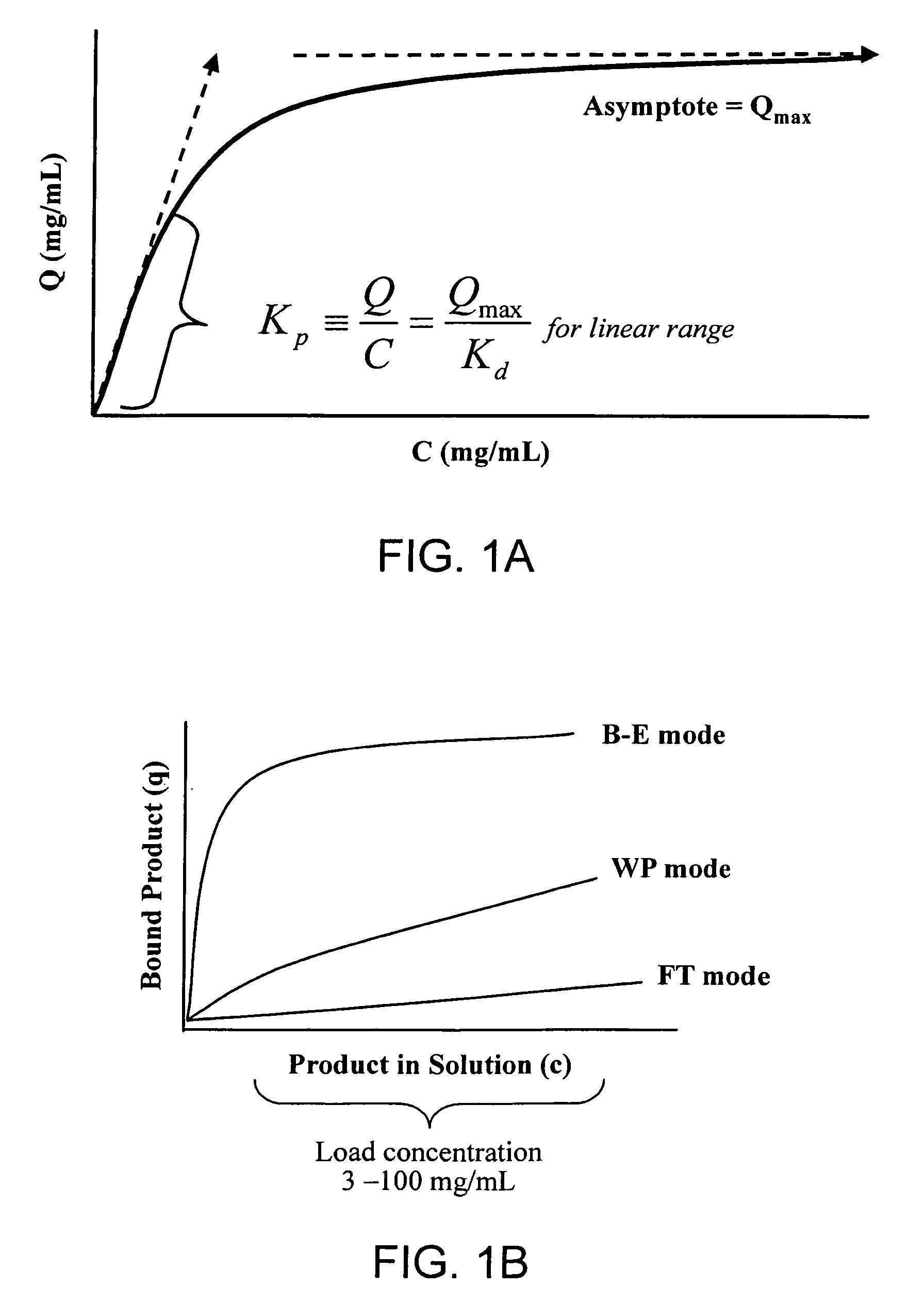
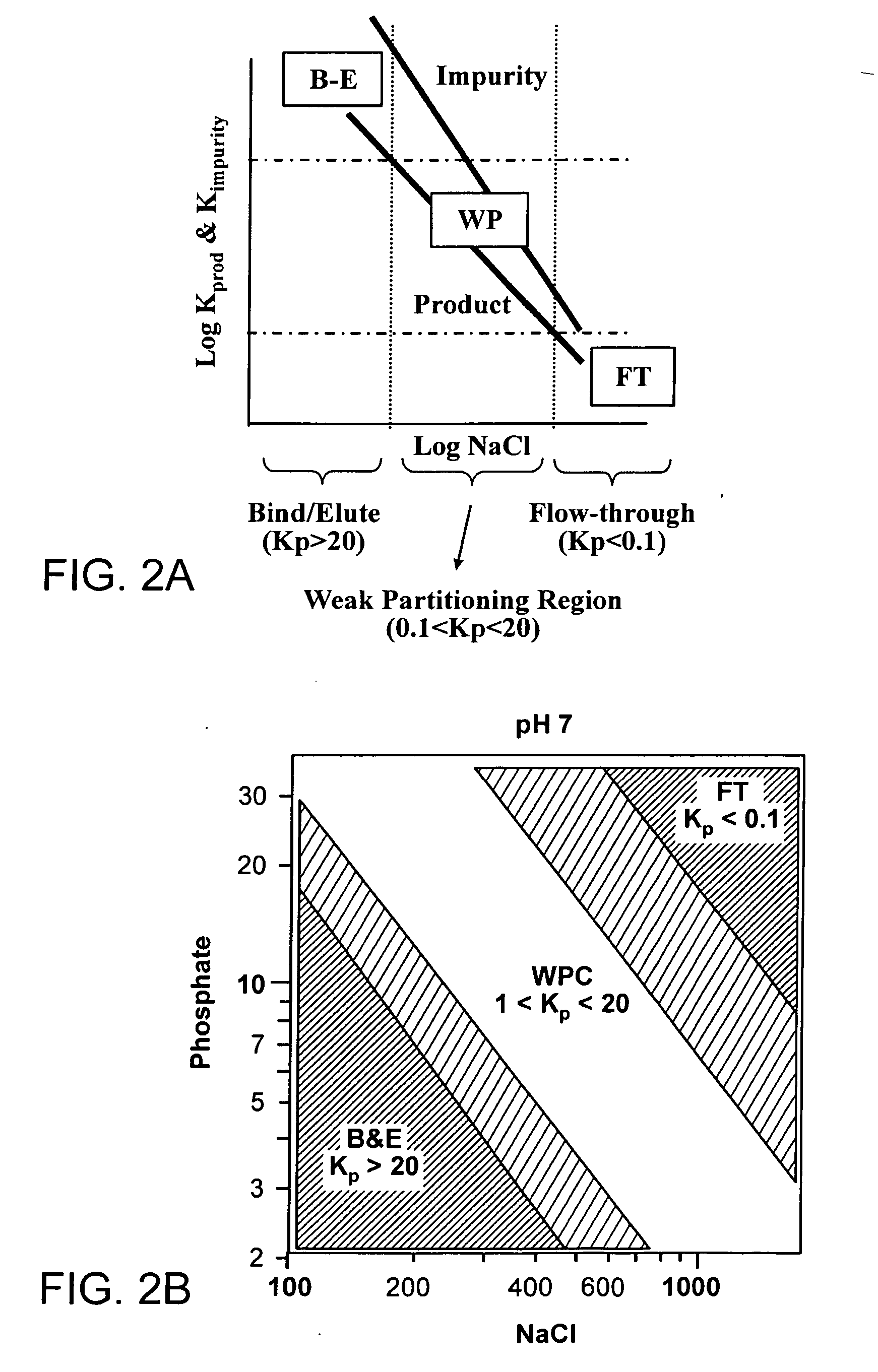
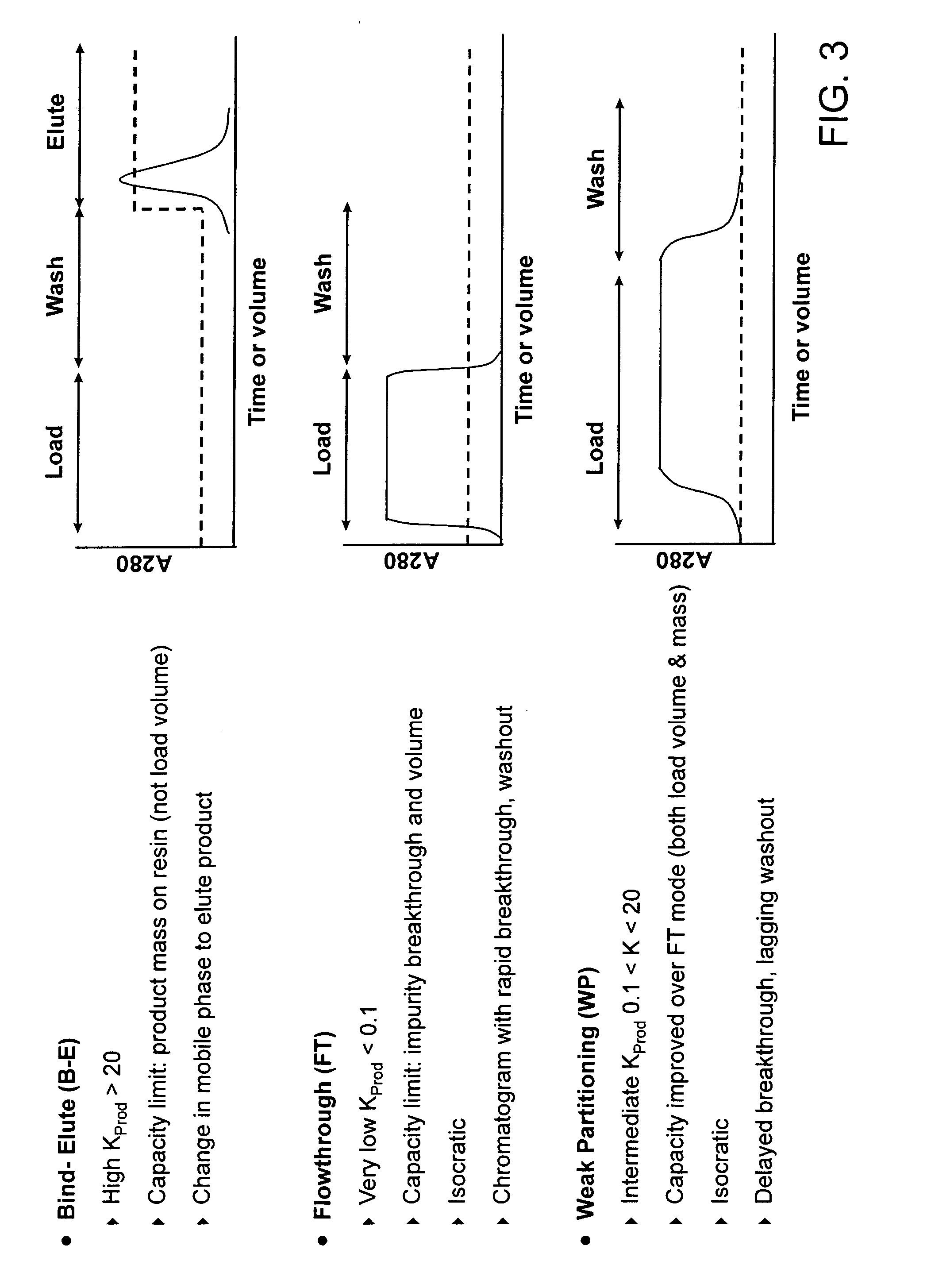
Method of weak partitioning chromatography
a chromatography and weak separation technology, applied in the field of recovering a purified product from a load fluid, can solve the problems of yield, purity and throughput, plague the manufacturing sector, and none of the conventional bind-elute or flow-through methods in the prior art can meet the needs of the biotechnology industry in terms of ionic strength, and achieve the effect of low concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1.1
t Screen to Establish WP and FT Conditions
[0123] A high throughput screen (HTS) was performed to identify the weak partitioning and flow-through conditions for Mab-AAB with TMAE-HiCap (M) medium. This screen varied the concentration of sodium chloride and pH to determine their effect on the extent of binding of MAB-AAB and process related impurities (Protein A and HCP) to the TMAE medium.
[0124] 50 μL of TMAE HiCap medium was added to each well of a 96 well filter plate. Each well was equilibrated in solutions made up 50 mM glycine and a variable amount of Tris buffer (depending upon the amount needed for neutralization to the pH specified in Table 1.1.1) and sodium chloride (specified in Table 1.1.2). The pH ranged from 7.6 to 9.0 and the sodium chloride ranged from 0 mM to 80 mM.
[0125] The buffer solutions used in each row were diluted on an automated pipetting system (Tecan 100 RST). The stock solution for the buffers were made from 500 mM glycine acidified with HCl to pH 3.0, a...
experiment 1.2
derflow-Through Conditions
[0132] The following experiment was performed in the flow-through (FT) mode, where the Mab-AAB interacts only very weakly with the column. Two runs were performed with load challenges of 110 mg / ml and 200 mg / ml of resin.
[0133] For all TMAE (HiCapM) anion exchange chromatography runs described in the Series 1 experiments, the following conditions were used (exceptions are noted in the individual experimental descriptions).
[0134] Operational flow rate—150-300 cm / hr
[0135] Equilibration 1-50 mM Tris, 2.0 M NaCl, pH 7.5 (5 column volumes)
[0136] Equilibration 2—as specified, approximately equivalent to the load pH and chloride content
[0137] Post load wash—as specified, approximately equivalent to the load pH and chloride content
[0138] Strip buffer—50 mM Tris, 2.0 M NaCl, pH 7.5 (5 column volumes)
Mabselect Protein A Chromatography
[0139] The culture containing the monoclonal antibody was purified at Pilot scale using a MabSelect column (2,389 mL) connected...
experiment 1.3
der Weak Partitioning Conditions (High Product Challenge)
TMAE (HiCap M) Anion Exchange Chromatography
[0142] Several Mabselect Protein A runs were performed essentially as described in Experiment 1.2 to generate the load material for these runs. The partially purified antibody pool from the Protein A step was further purified over the TMAE column. The load to the TMAE column was in 50 mM Tris, pH 8.2. The column diameter was 0.5 cm and the bed height was 10 cm bed height (volume—2.0 mL). The column was challenged to a load of 500 mg / mL resin, with a load concentration of 27.7 mg / mL.
[0143] The column was equilibrated with 5 column volumes of a solution containing 50 mM Tris, 2M NaCl pH 7.5 followed by another equilibration step comprising a 50 mM Tris, pH 8.2 solution. The column was then loaded to 500 mg product / ml resin with the neutralized Protein A peak from the previous step and the product was recovered in the column effluent during the load cycle and some column volumes of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| partition coefficient | aaaaa | aaaaa |
| partition coefficient | aaaaa | aaaaa |
| partition coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com