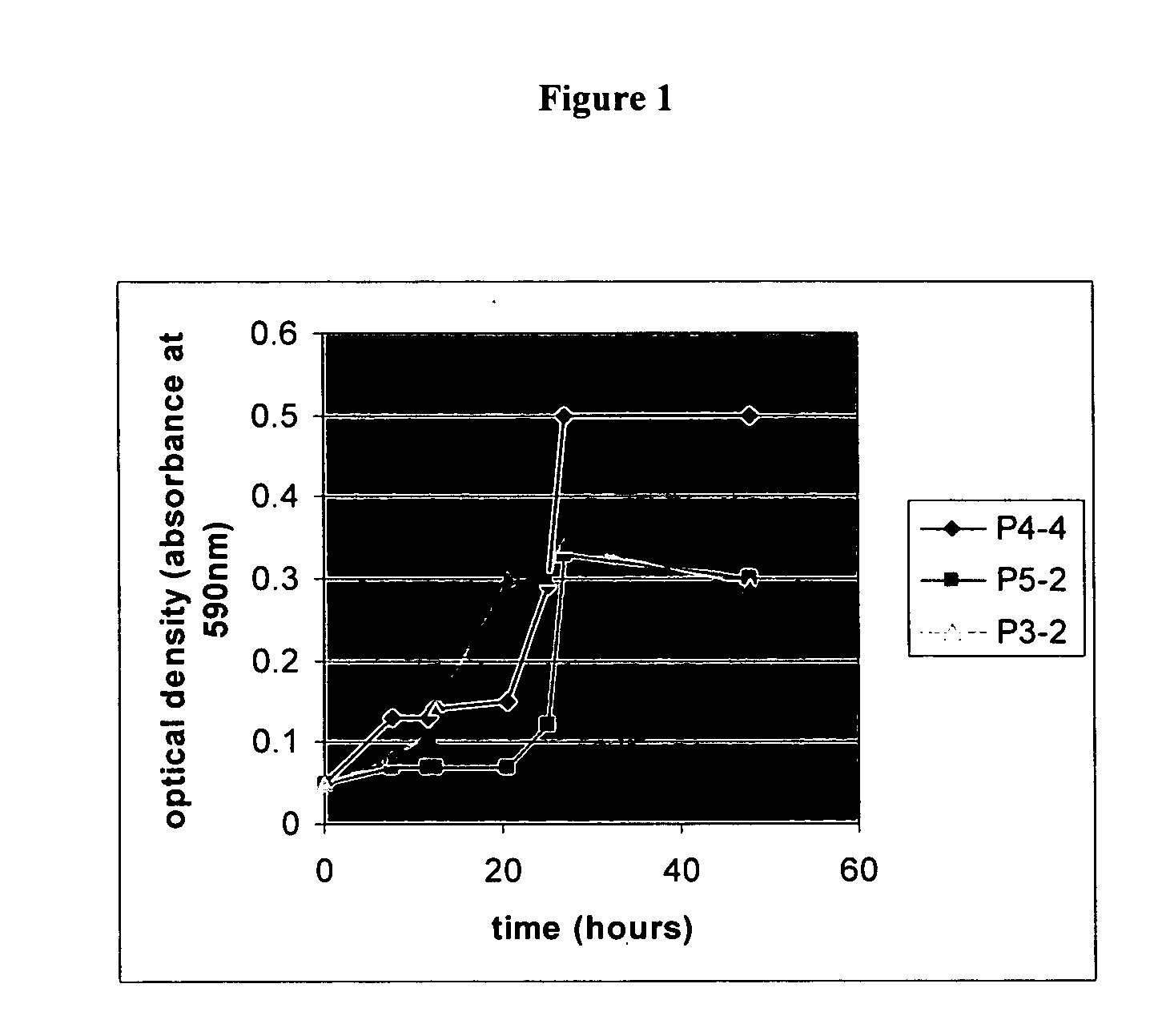
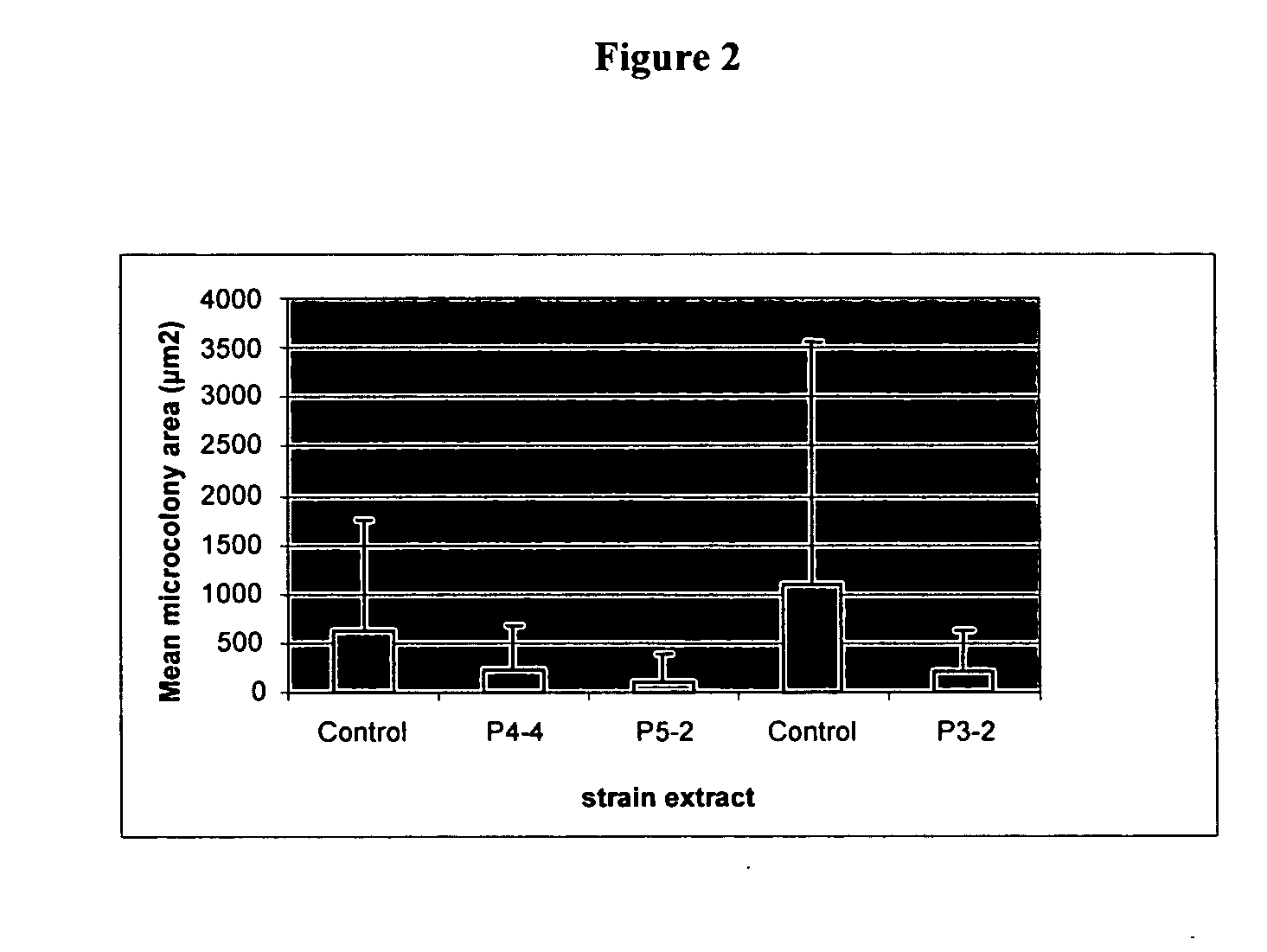
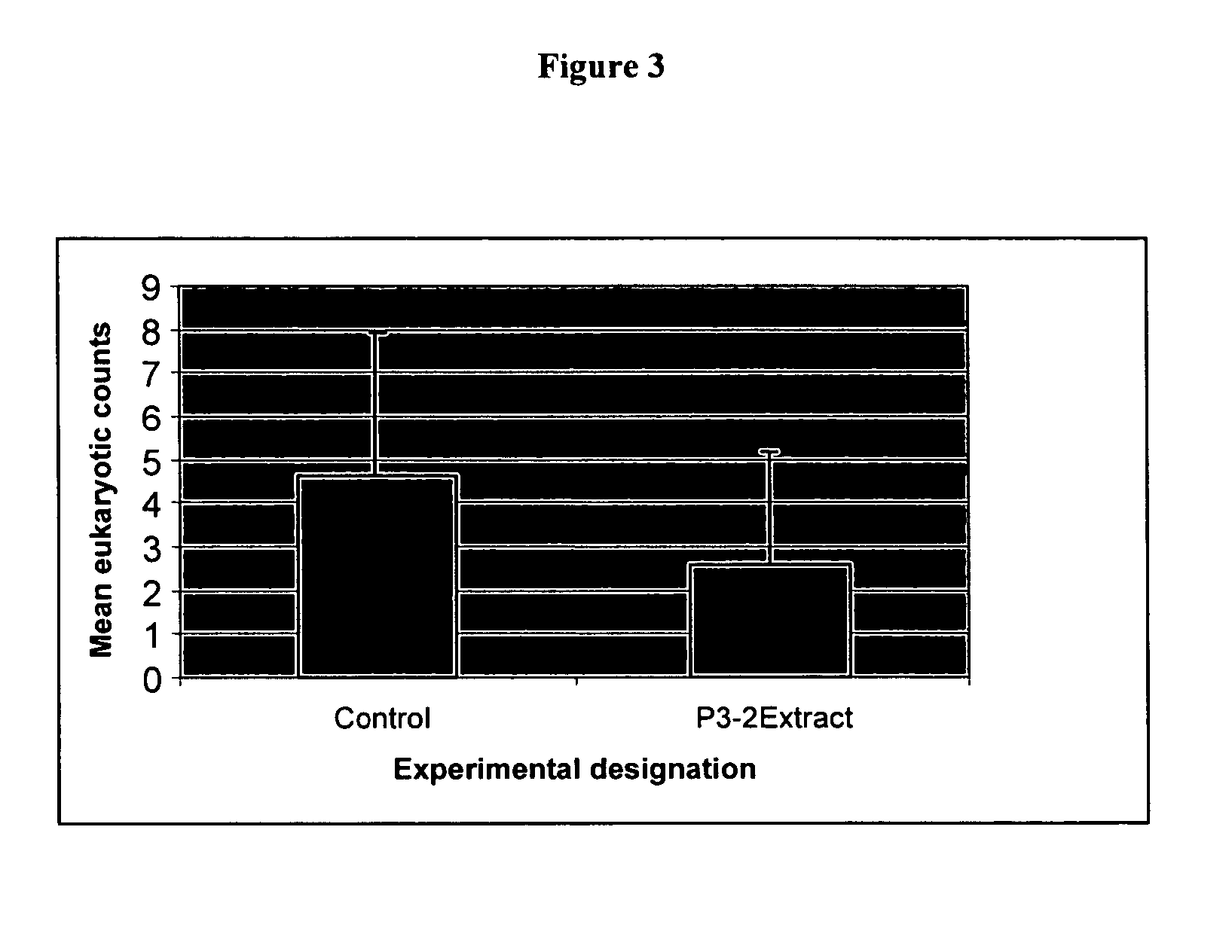
Antagonistic properties of reef fish microflora
a technology of anti-antagonistic properties and reef fish, which is applied in the direction of antibodies to medical ingredients, paints with biocides, bacteria based processes, etc., can solve the problems of prolonging the life of drugs and inability to develop resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0096] The reef fish, Sparisoma ninidae (Parrotfish) and Lujanus purpureus (Red Snapper) were caught above a coral reef in True Blue Bay, Grenada at N=11°059.908 and W=061°46.282 in accordance with a Global Positioning System (GPS) (GEKO 201 Garmin Taiwan). Fish were either trapped in a fishpot or shot with a spear gun. The fishpot used to trap the fish was made of 0.5 inch galvanized square mesh, 36 inches long, 16 inches wide and high. The cornucol hole (horn shaped) on one side of the fishpot was 7 inches in diameter on the outer surface and it tapered inside the fishpot to a 5 inch diameter. The fishpot was located approximately 10 feet deep, next to a coral reef, approximately 150 feet away from shore. At the surface after caught, each fish was washed twice with autoclaved artificial seawater to remove any loosely associated microbes and then immediately placed in a sterile plastic bag on ice. The fish was then transported back to the laboratory.
example 2
Isolation of Pure Cultures from the Epithelial Mucosal Surfaces of Coral Reef Fish
[0097] Normal fish microflora was collected from the mucus surface of the fish with a sterile cotton swab and plated on Artificial Sea Water Agar (ASWA) medium. ASWA medium was used to mimic fish mucus. Artificial Sea Water Agar (ASWA) contained (g / l) of solution: NaCl 21.10, KCl 0.58, CaCl2×2H2O 1.20, MgCl2×6H2O 4.73, NaHCO3 0.08, MgSO4×7H2O 2.63, yeast extract 10.00, malt extract 4.00, glucose 4.00, agar 15.00. Solution was adjusted to pH 7.5-8.0, autoclaved at 121° C. for 20 min, and poured into sterile Petri dishes. Artifical Sea Water (ASW) liquid broth was prepared as above except the 15.00 g of agar was omitted.
[0098] Plates were incubated at 29° C. for 48 hours. Separate colonies were picked, inoculated and grown in the same liquid medium and cultured under the same temperature for 48 hours. Then the cultures were plated again on the solid ASWA. Gram staining was done to ensure that cultures ...
example 3
Morphological and Physiological Characterization of Isolates
[0101] Morphology of the cells from the isolates was studied after Gram-staining using phase contrast microscopy. Blood agar (Anon, 1953), Mannitol salts agar (Anon, 1953), Mac Conkey Agar (Anon, 1953), and Cystein tryptic agar (Anon, 1953) were used to test type of hemolysis, acid production from mannitol, resistance to bile salts, and oxidation / fermentation of glucose, respectively. Catalase reaction and oxidase reaction were performed using traditional techniques known in the art.
[0102] Twelve of the fifteen parrot fish isolates were gram positive, eight were cocoidal, and ten were pigmented. Three of the five snapper isolates were gram positive, four were cocoidal, and four were pigmented. The majority of the isolates were gram positive and pigmented (Table 2). Most of the isolated organisms were aerobic, heterotrophic, halotolerant, and mesophilic (Table 3). All of them grew best at 28° C. and salinity of 40 ppt, how...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com