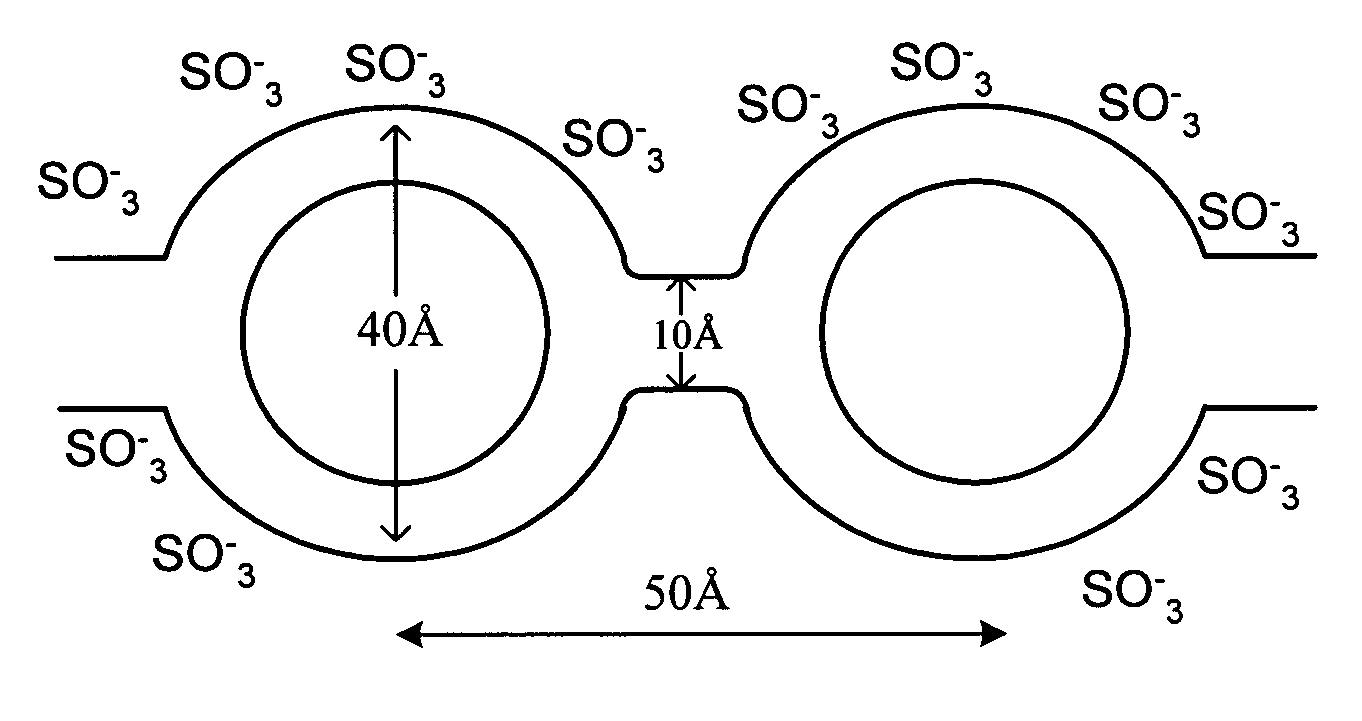
Advanced solid acid electrolyte composites
a solid acid electrolyte and composite technology, applied in the direction of cell components, electrochemical generators, cell component details, etc., can solve the problems of irreversible degradation, limited device operation efficiency, and degradation of the conductivity of the electrolyte, so as to improve the mechanical properties and mechanical properties. , the effect of high protonic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of CsH2PO4:LaPO4*nH3PO4 Solid Acid Composite
[0147] Solid acid CsH2PO4 was prepared according to a reported method (Boysen, D. A., et al. Science, 2004, 303, 68-70). LaPO4 was purchased from Alpha Aesar Company. Solid acid composite CsH2PO4:LaPO4*nH3PO4 was prepared by mechanically mixing a 50:50 mixture by mole fraction of CsH2PO4 and LaPO4*nH3PO4 using a mortar and a pestle at about 23° C. under an ambient pressure. The composite material was used without further purification.
example 2
Comparison of Conductivity of Pure Solid Acid and Composite
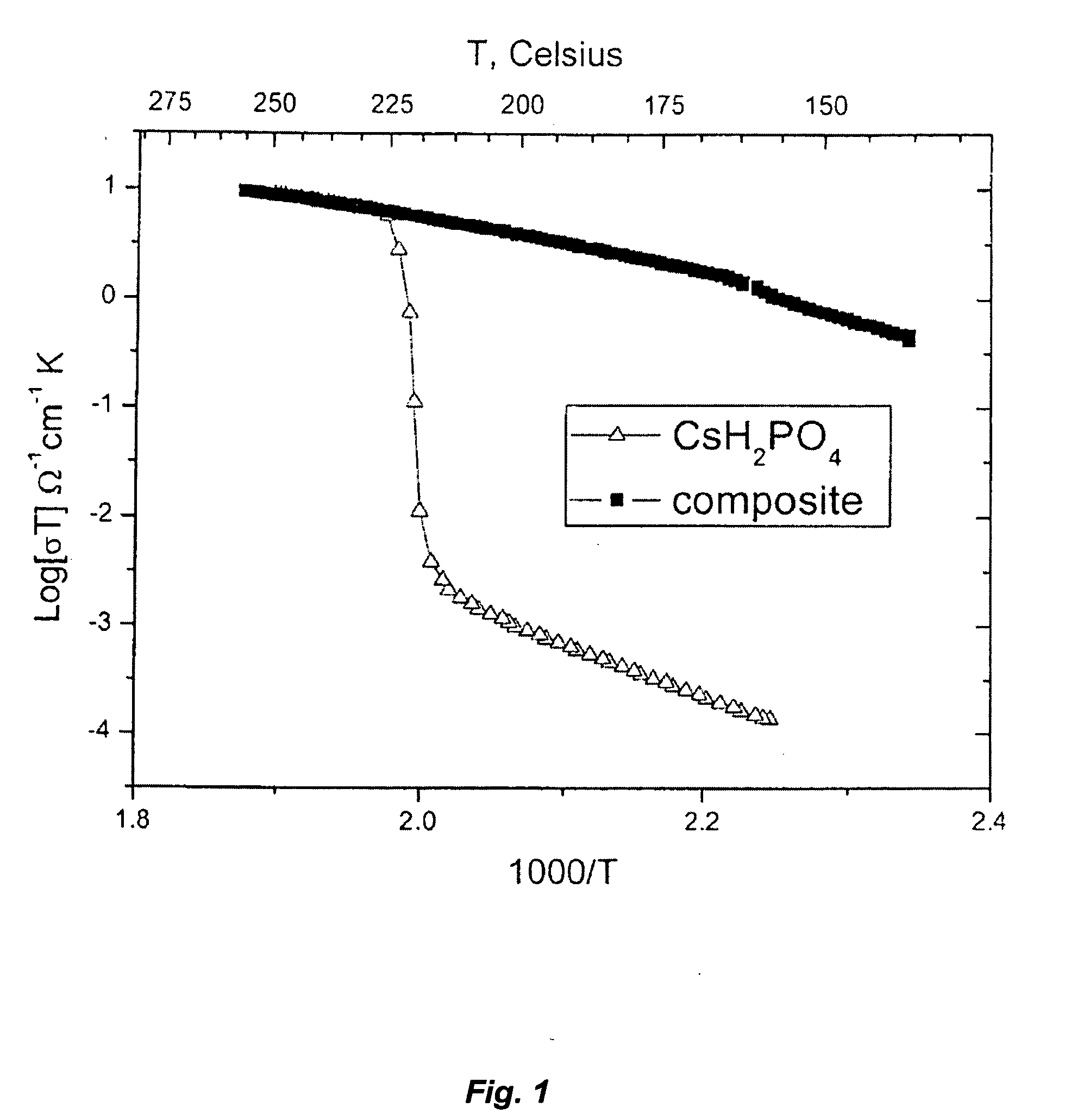
[0148]FIG. 1 shows a comparison of the conductivity of pure CsH2PO4 versus a CsH2PO4 / LaPO4*H2O(H3PO4)g composite material. The composite has higher or equal conductivity to that of pure CsH2PO4 at all measured temperatures. Measurements were taken upon heating and cooling at 1° C. / min, under flowing air atmospheres with a water partial pressure ˜0.4 atm.
example 3
Stability of Solid Acid Composite
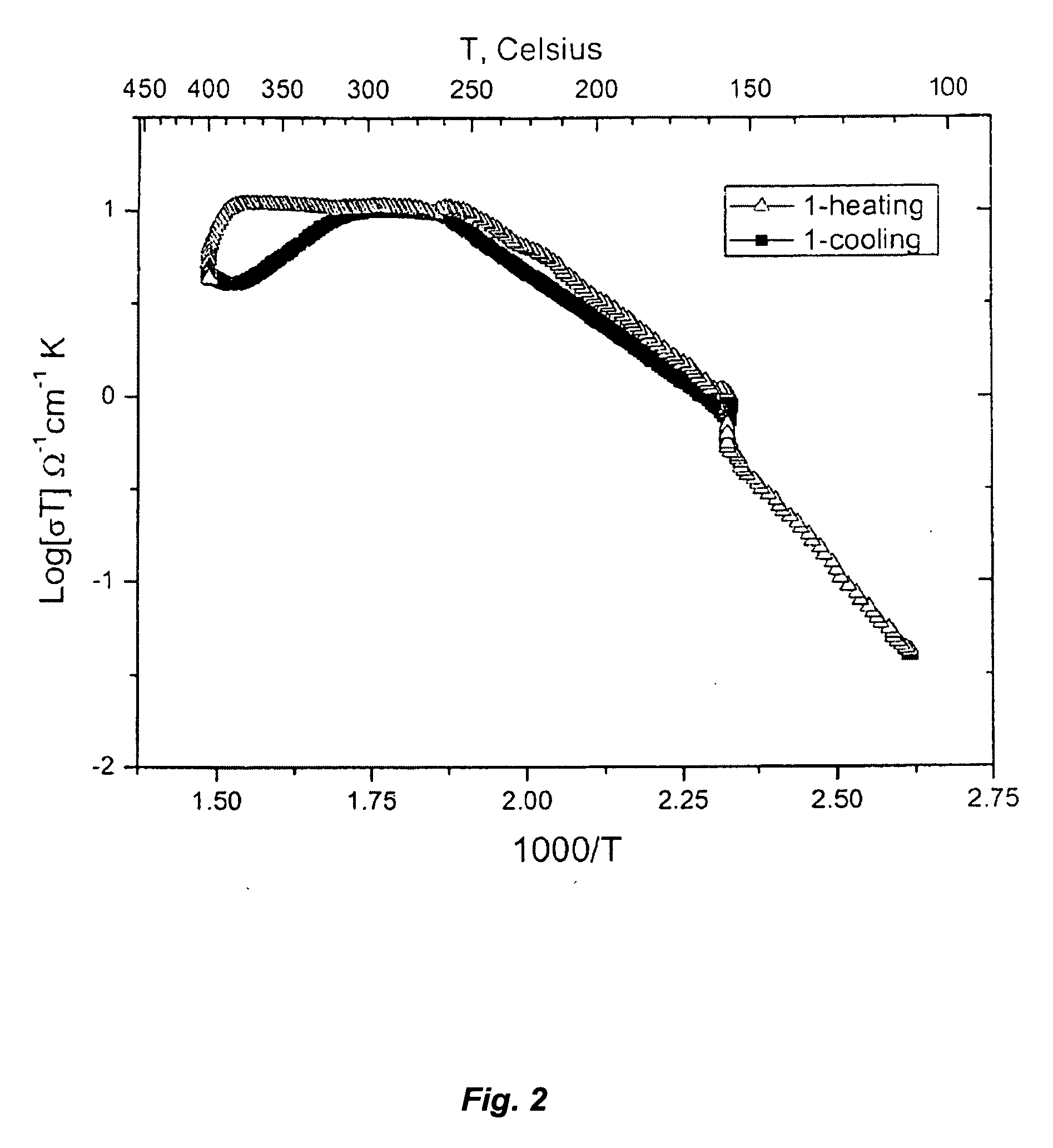
[0149]FIGS. 2 and 3 illustrate the stability of solid acid composite and the rehydrating of the solid acid composite. The conductivity of the solid acid composite CsH2PO4 / LaPO4*H2O(H3PO4)g can be measured up to 400° C., whereas a sample of pure solid acid CsH2PO4 would melt at ˜330° C., resulting in a short circuit in the experimental setup used. All measurements were taken with heating / cooling rates of 1° C. / min, under flowing air atmospheres with a water partial pressure ˜0.4 atm. The solid acid composite CsH2PO4 / LaPO4*H2O(H3PO4)g also has the ability to rehydrate at 156° C. after having been dehydrated at 400° C. for 6 hrs, a property not seen in CsH2PO4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| conductivity | aaaaa | aaaaa |
| proton conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com