Processes for the preparation of isocyanates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
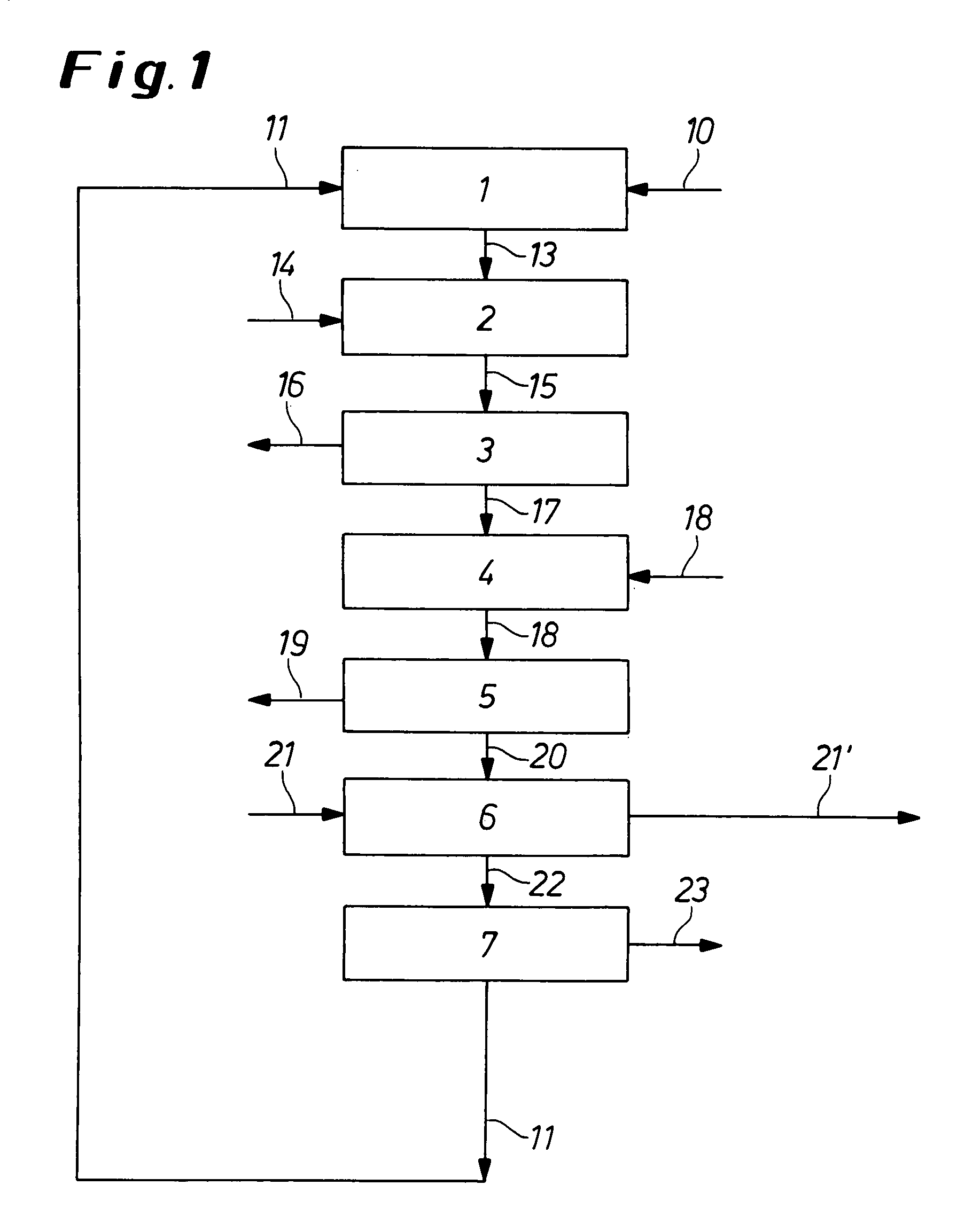
[0071] A process according to an embodiment of the invention is explained in more detail with reference to FIG. 1. FIG. 1 is a representative flowchart depicting a process according to the invention for the preparation of TDI.
[0072] In a first stage 1 of the preparation of isocyanates, chlorine 11 is reacted with carbon monoxide 10 to give phosgene 13. In the following stage 2, phosgene 13 from stage 1 is reacted with an amine 14 (in this case toluenediamine) to give a mixture 15 of isocyanate (toluene-diisocyanate, TDI) and hydrogen chloride, the isocyanate 16 is separated off (in stage 3) and utilized. The HCl gas 17 is reacted with oxygen 18 in the HCl oxidation process 4.
[0073] For this e.g., a UV-transparent reaction tube can be used, this being charged with HCl and O2 in the stoichiometric ratio of 4:1. The reaction mixture is irradiated in the reaction tube with short wavelength (<250 nm), coherent UV light with the aid of a pulsed excimer laser. The temperature of the reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com