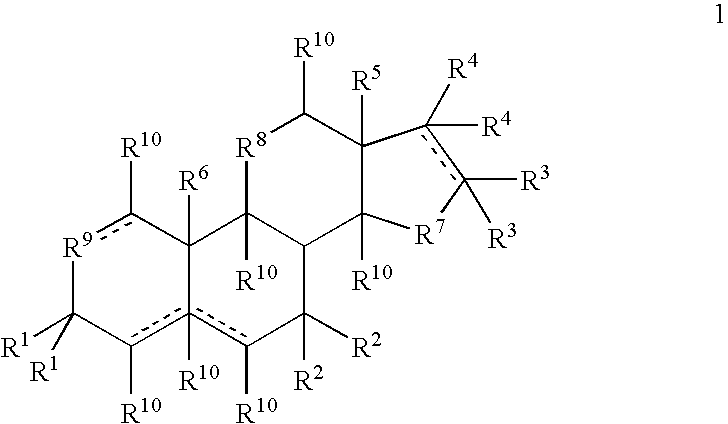
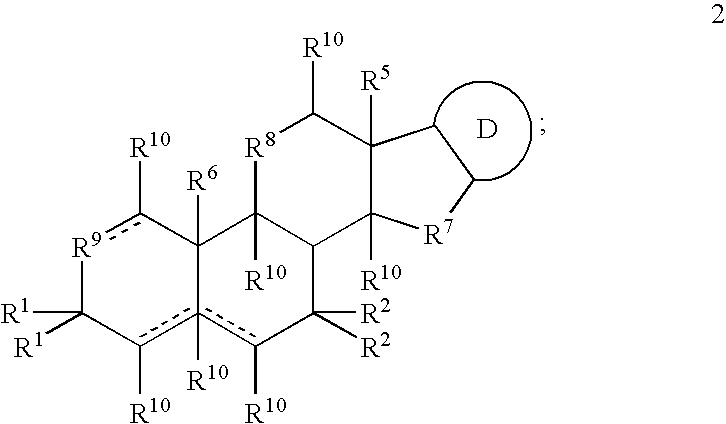
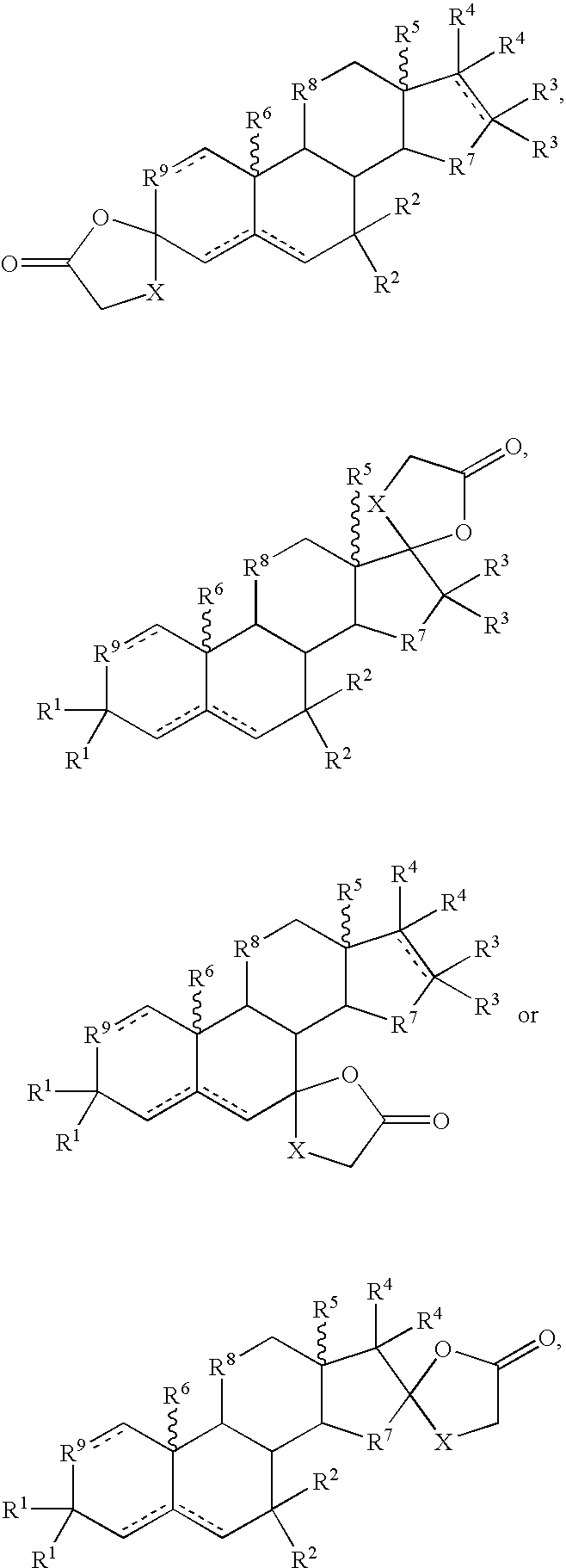
Formulations and Treatment Methods
a technology of th1 and th2 receptor, applied in the field of making and using steroid, can solve the problems of impaired production, undetectable or suboptimal properties of compositions, and general inability to prefer or suitable parenteral excipients such as dioxane, dmso or chloroform, so as to facilitate th1 immune response, enhance the expression of one or more cytokines, and reduce the expression of on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
BrEA Formulation
[1071] Two lots of a non-aqueous BrEA formulation were made at a BrEA concentration of 50 mg / mL in 25% polyethylene glycol 300, 12.5% dehydrated ethyl alcohol, 5% benzyl benzoate, and 57.5% propylene glycol as follows. BrEA was obtained from Procyte, Inc. The remaining excipients are shown below.
SupplierFinal ProductExcipientSpecificationLot No.ConcentrationPropylene glycolUSPArco Chemical57.5% (v:v)HOC-61220-01104Polyethylene glycolNFUnion Carbide 25% (v:v)300695752Dehydrated alcoholUSPMcCormick12.5% (v:v)Distilling 97K10Benzyl benzoateUSPSpectrum 5% (v:v)PharmaceuticalsMG025
[1072] The formulation was prepared by suspending BrEA in polyethylene glycol 300, and sequentially adding propylene glycol, benzyl benzoate, and dehydrated ethyl alcohol to form a solution, which was diluted to the final desired volume with additional propylene glycol. The procedure is described below.
[1073] The calculated amount of polyethylene glycol 300 was added to a compounding vesse...
example 2
BrEA Drug Substance and BrEA Formulation Stability
[1076] An accelerated stability study of 6 months duration is conducted using BrEA and the formulations from example 1. Samples are taken at 1, 2, 3, 4, 5, and 6 month time points and compared with the specifications listed in example 1. Real time stability (25° C., 60% relative humidity) is conducted using BrEA formulation Lots 1 and 2, with sampling time points at 3, 6, 9, 12, 18, 24, and 36 months. After 3 months of storage at 40° C. and 75% relative humidity, the assay potency of BrEA is at least 95% of the label value. The results from the stability testing indicate that BrEA is stable for at least 3 months at elevated temperature and humidity in the Lot 1 and 2 formulations.
example 3
Primate Intermittent Dosing Protocol
[1077] Pig-tail Macaque monkeys infected with the SHIV229 retrovirus were treated with a BrEA formulation as described in example 1. SHIV229 is a recombinant retrovirus containing HIV and SIV sequences. J. Thompson et al., abstract #75, 16th Annual Symposium on Nonhuman Primate Models for AIDS, Oct. 7-10, 1998, Atlanta, Ga., M. Agy et al., abstract #67, 16th Annual Symposium on Nonhuman Primate Models for AIDS, Oct. 7-10, 1998, Atlanta, Ga. In monkeys, it establishes an aggressive infection that leads to severe symptoms of end-stage disease in infected untreated animals at about 180-210 days after infection. Four pig-tail macaques (2 / group) received subcutaneous injections of the formulation at 1 or 2 mg / kg body weight for 10 consecutive days (Protocol 1). On week 8, 3 of the 4 monkeys were retreated and 2 treatment naïve monkeys were treated with 5 mg / kg of the formulation on an every other day basis for a period of 20 days (Protocol 2). On week...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com