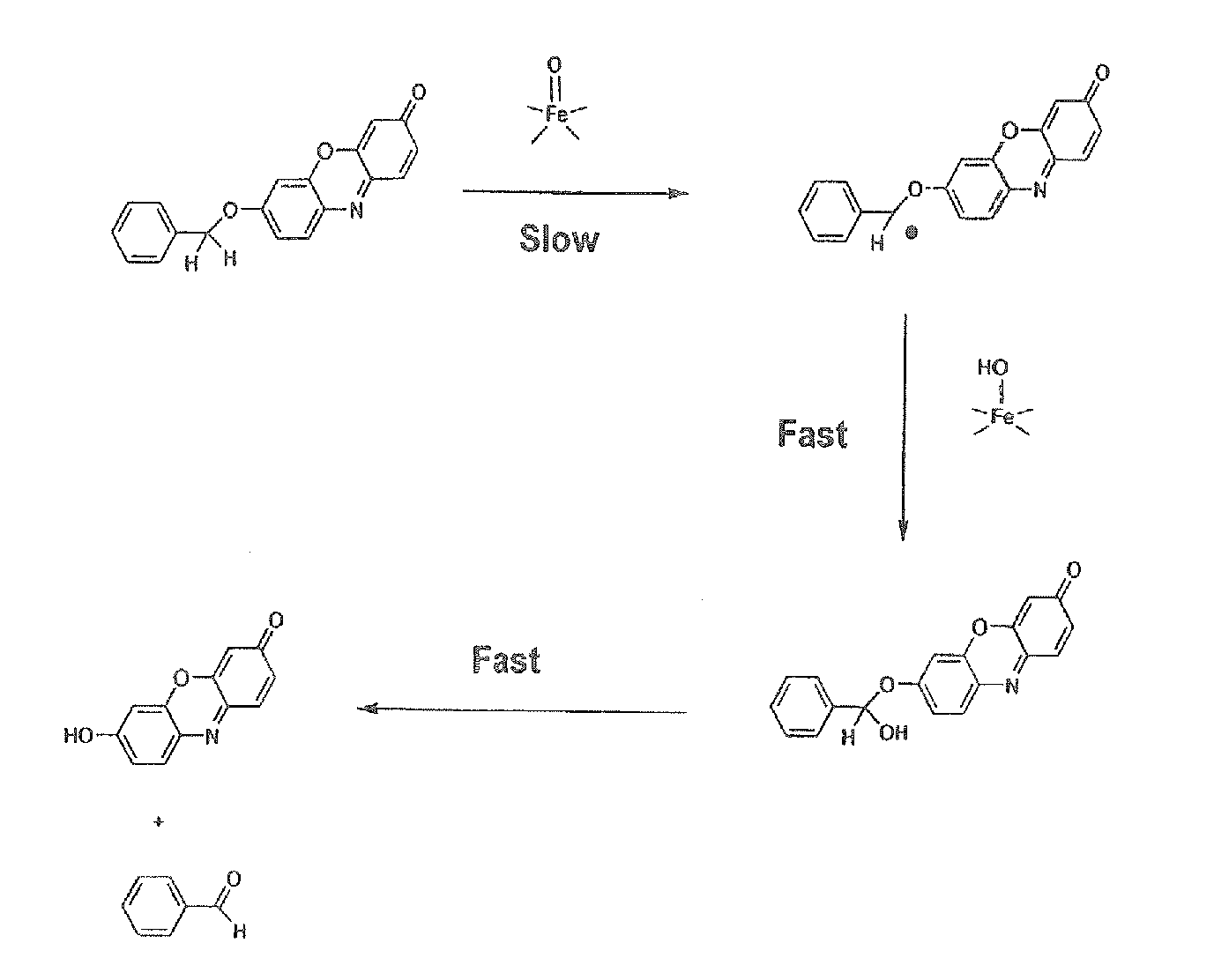
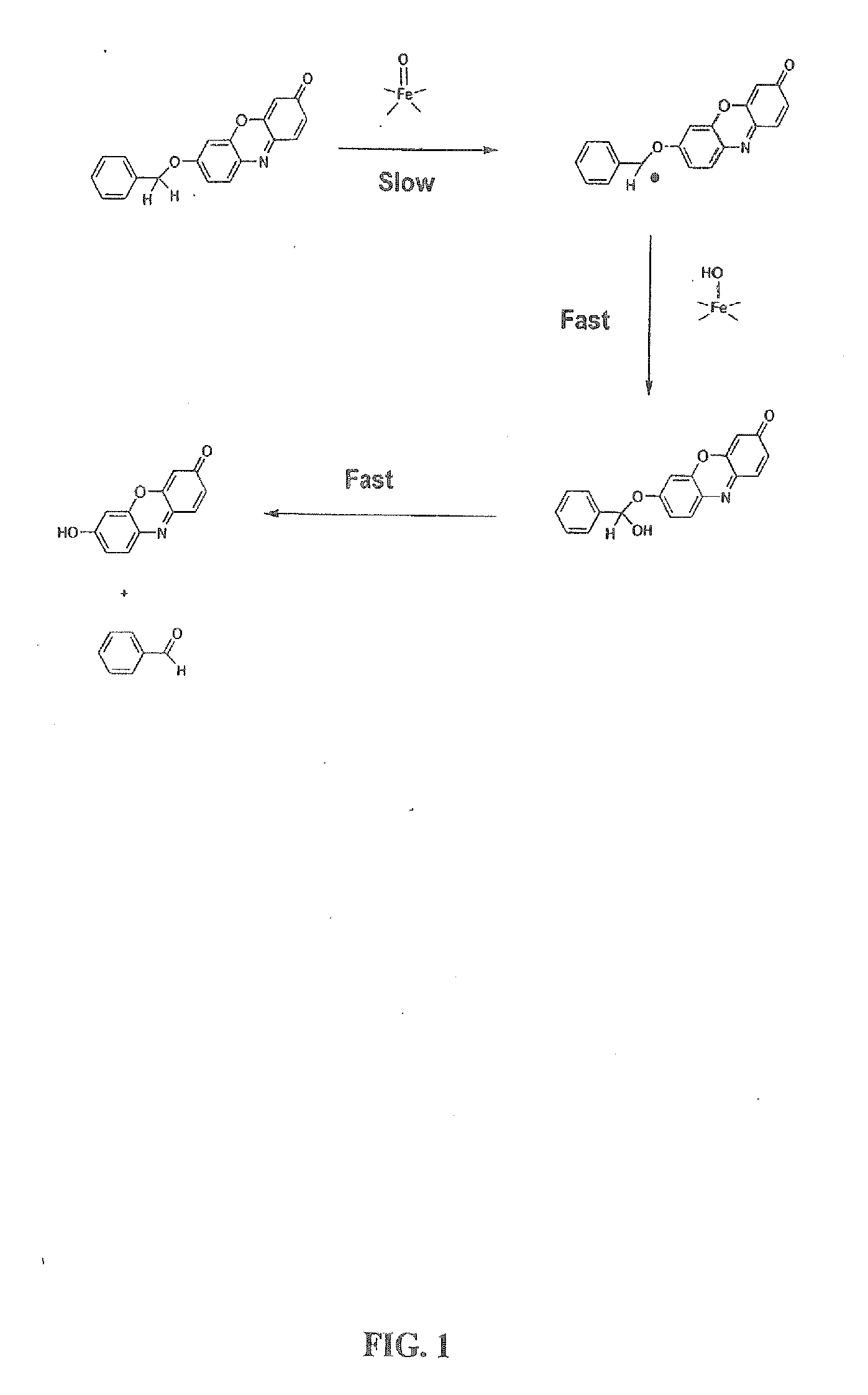
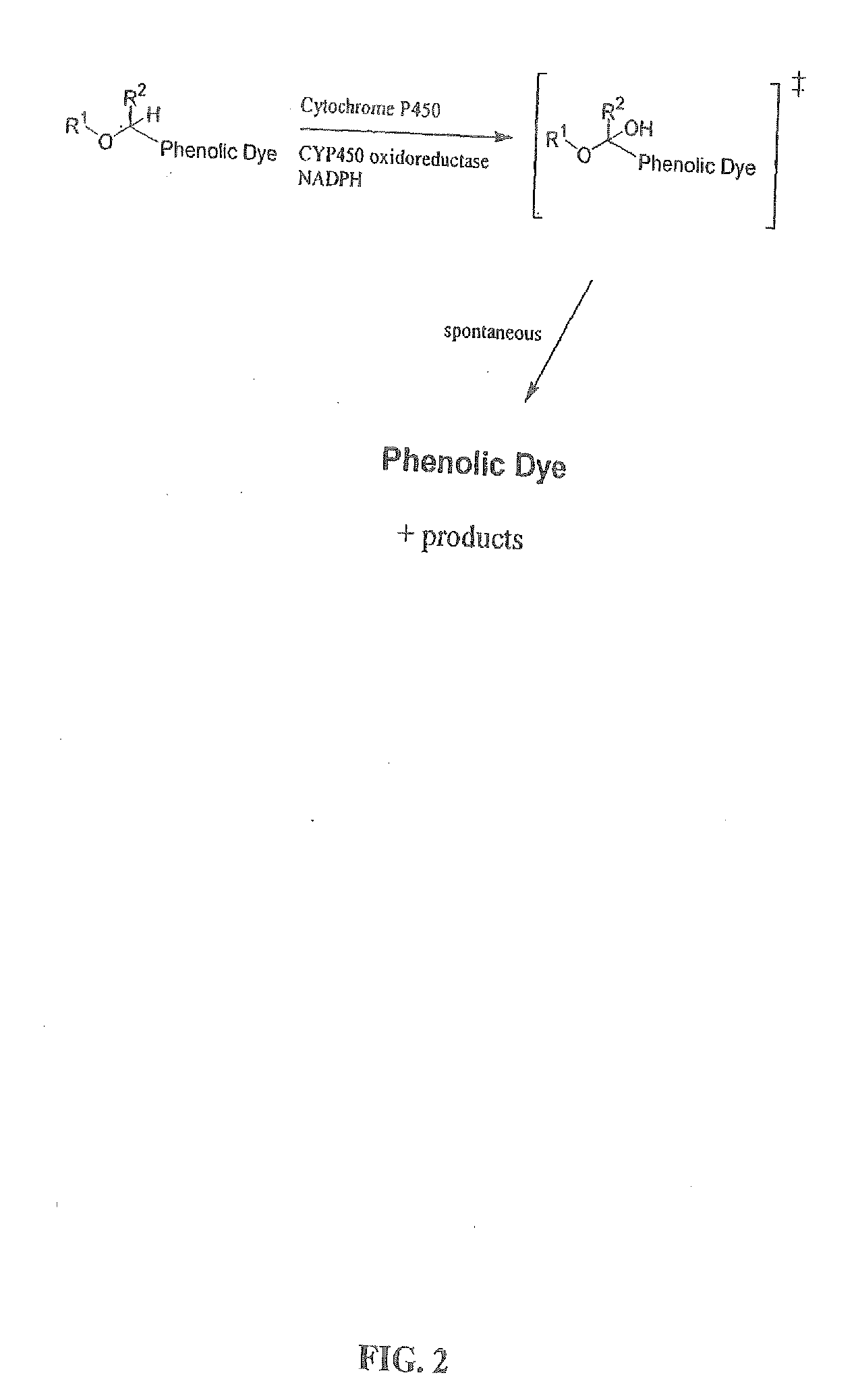
Optical molecular sensors for cytochrome P450 activity
a technology of cytochrome p450 and molecular sensors, which is applied in the field of optical fluorogenic indicators of cytochrome p450 activity, can solve the problems of undesirable drug-drug interaction, poor pharmacokinetic properties, and reduced drug development costs and/or increased profits from earlier market entry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Fluorogenic Substrates for CYP450
Preparation of Benzyloxymethylresorufin (BOMR)
[0094]For all syntheses of the compounds of the invention, as herein described in this and the following EXAMPLES, the following protocols were (or are, with respect to EXAMPLE 7) followed unless so stated: Reaction conditions were (or are) carried out under atmospheric nitrogen. All solvents utilized were dried over 3 Å molecular sieves. All chemicals and reagents were used as purchased without further purification unless stated. Benzylchloromethylether was purchased from Fluka Chemie AG, Resorufin was purchased form Aldrich Chemical Co., 7-Hydroxy-3-trifluoromethycoumarin and 7-Hydroxy-3-cyanocoumarin were purchased from Molecular Probes and all were used as received. See also Wolfbeis, Otto, Z. Naturforsch. (1977) 32a, 1065-1067. Column chromatography was executed with J. T. Baker silica gel (particle size=0.04-0.061 mm) using solvent combinations determined via initial TLC analysis with M...
example 2
Preparation of 7-Benzyloxymethyloxy-3-cyanocoumarin (BOMCC)
[0096]7-Benzyloxymethyloxy-3-cyanocoumarin (BOMCC) was prepared as follows: A mixture of 7-Hydroxy-3-cyanocoumarin1, (187 mg, 1 mmol) and K2CO3 (248 mg, 1.5 mmol), in DMF (15 mL) was vigorously stirred at 0° C. for 25 min. Benzylchloromethylether (2.32 mL, 10.0 mmol), was then added quickly to the reaction. The bright yellow mixture was stirred at 0° C. for 45 min. After which time the reaction turned to a colorless solution. The reaction was monitored to completeness by TLC(Rƒ0.5, 1:1 EtOAc:Hex.and Rƒ0.24, CHCl3). The reaction was then brought up in Et2O (35 mL), and extracted with saturated NaHCO3 (30 mL). The aqueous layer was extracted two more times with Et2O (30 mL). The etheral layer was then combined then dried with anhydrous NaSO4 and evaporated under reduced pressure. Chromatography of the crude product on silica gel (gradient 0-5% MeOH in CHCl3) gave the pure 7-Benzyloxymethyloxy-3-cyanocoumarin as a white solid (...
example 3
Preparation of 7-(p-methoxybenzyloxy-4-trifluorocoumarin (MOBFC)
[0097]7-(p-methoxybenzyloxy-4-trifluorocoumarin (MOBFC) was prepared as follows: A mixture of 7-Hydroxy-3-trifluoromethycoumarin, (230 mg, 1 mmol), K2CO3 (248 mg, 1.5 mmol), and KI (1.66 g, 10 mmol) in DMF (15 mL) was vigorously stirred at 25° C. for 25 min. Paramethoxybenzylchloride (1.35 mL, 10.0 mmol), was then added quickly to the reaction. The bright yellow mixture was stirred at 25° C. for 1 hr. After which time the reaction turned to a colorless solution. The reaction was monitored to completeness by TLC(Rƒ=0.67, 1:1 EtOAc:Hex.and Rƒ0.3 CHCl3). The reaction was then brought up in Et2O (35 mL), and extracted with saturated NaHCO3 (30 mL). The aqueous layer was extracted two more times with Et2O (30 mL). The etheral layer were combined then dried with anhydrous NaSO4 and evaporated under reduced pressure. Chromatography of the crude product on silica gel (gradient 0-5% MeOH in CHCl3) gave the pure 7-Paramethoxybenz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical structure | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com