Method for the Simultaneous Determination of Blood Group and Platelet Antigen Genotypes
a blood group and platelet antigen technology, applied in the field of ultra high throughput (uht) multiplex pcr genotyping method, can solve the problems of high cost of blood collection facility, single-test drive method, and blood collection facility routinely performed method that is very cost ineffective,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
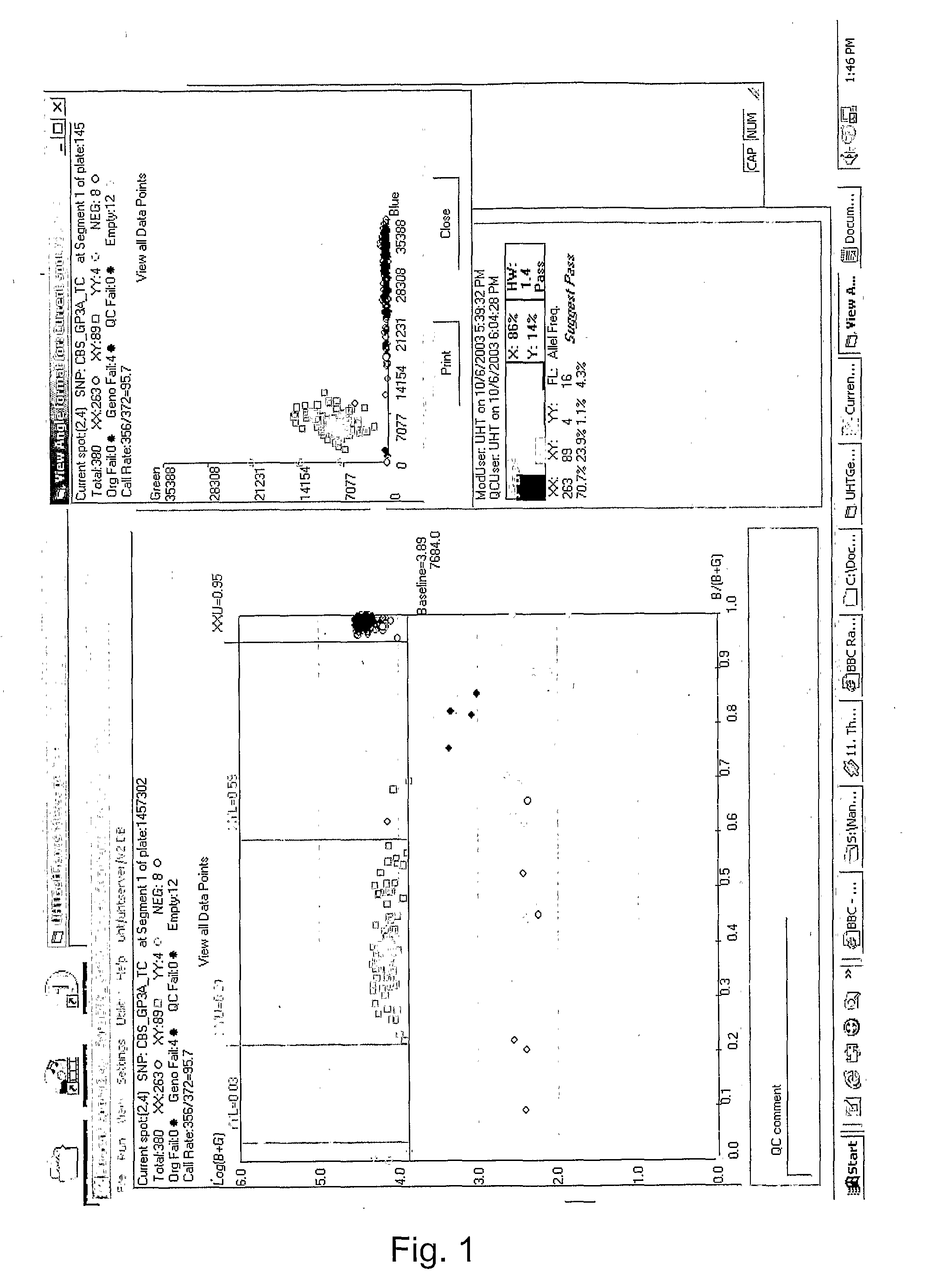
[0057]A preferred protocol for the multiplex blood group and HPA SNP Genotyping is provided. Although the present example analyzes 12 SNP extension primers, the present invention is not limited to the analysis of a maximum of 12 SNPs, but may include a plurality of SNPs relating to more than one or all of the blood group or HPA SNPs.
[0058]Additional blood group and platelet antigen SNPs for clinically relevant antigens embodied by the present invention appear in Table 1A. Primer pairs and probes, such as those exemplified in Tables 1 and 2, corresponding to these SNPs of clinical relevance, can be prepared according to the teachings of the present invention. Target primers may be initially identified from existing databases (e.g. autoprimer.com) based on information corresponding to the SNP of interest and the corresponding flanking regions, and subsequently optimized as herein disclosed for use in accordance with the present invention.
I (a). PCR Primer PoolingStepAction1Dilute each...
example 2
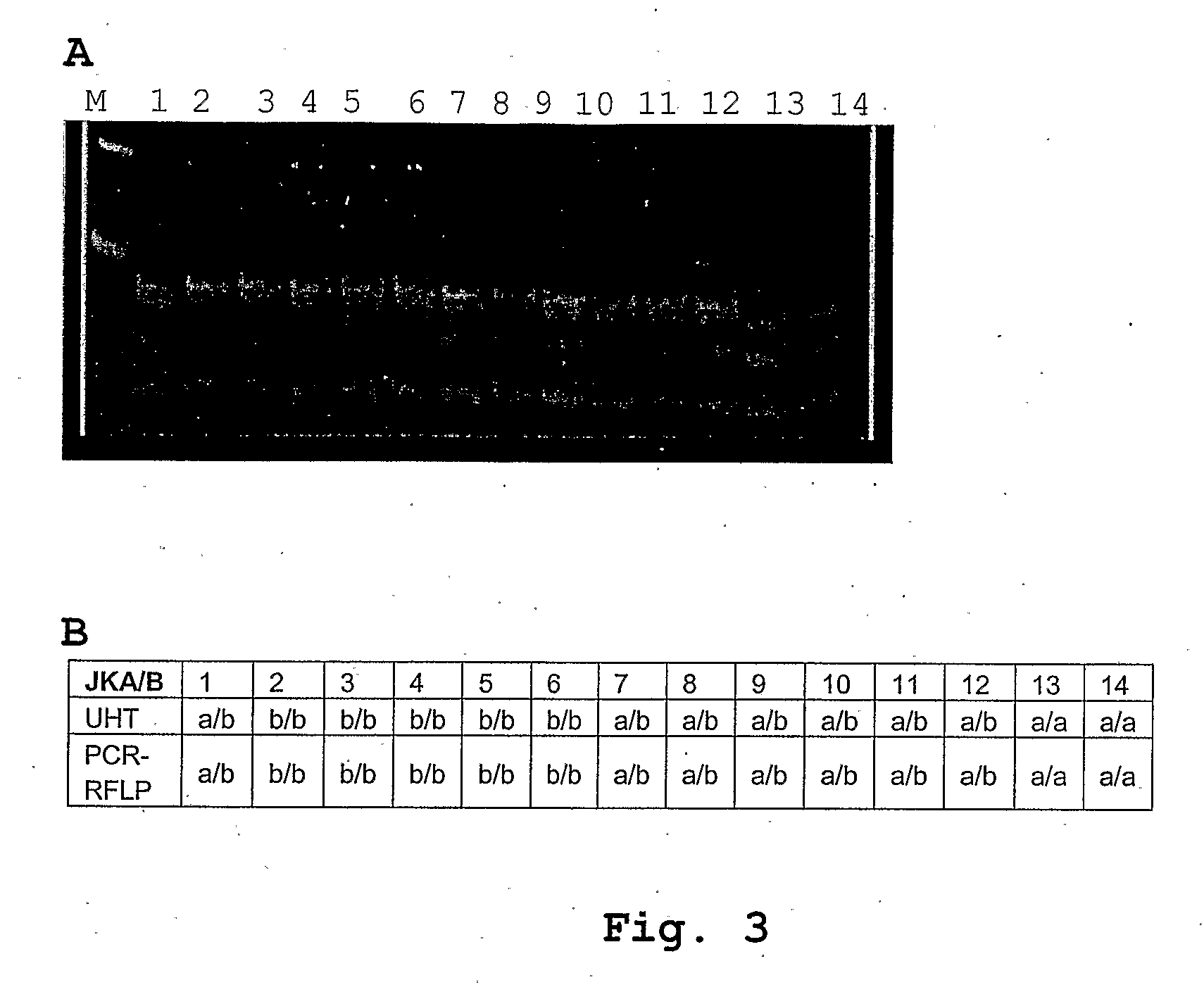
[0067]However, it should be obvious to one skilled in the art that other methodologies and / or technologies for SNP identification could be used, providing that the novel DNA sequences disclosed above are also used. Other embodiments could include the following but without limitation to micro-arrays on glass slides or silica chips, the use of mass spectrometry, or oligo-ligation and extension techniques to detect the SNPs of interest.
[0068]A preferred method of the present invention relates to a method for the detection of blood group and HPA genotypes. The present invention also provides novel DNA sequences that are used as primers in a multiplex PCR format according to the present invention to amplify the genomic regions of interest. The present invention also provides novel combinations of DNA sequences that are used in said multiplex PCR format, and for novel DNA sequences that are used to detect blood group and platelet SNPs.
[0069]A preferred application of the present invention...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| Rh | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com