Water soluble magnesium compounds as cleaning agents and methods of using them
a technology of water soluble magnesium and cleaning agents, applied in detergent compounding agents, water softening, inorganic non-surface active detergent compositions, etc., can solve the problems of hard water spotting, filming, staining or soap scum left on the surface, lack of other materials commonly used in cleaning compositions, etc., to reduce or prevent the effect of reducing the formation of lime scal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Water Soluble Magnesium Compounds Reduce Precipitation of Calcium Salts from Hard Water
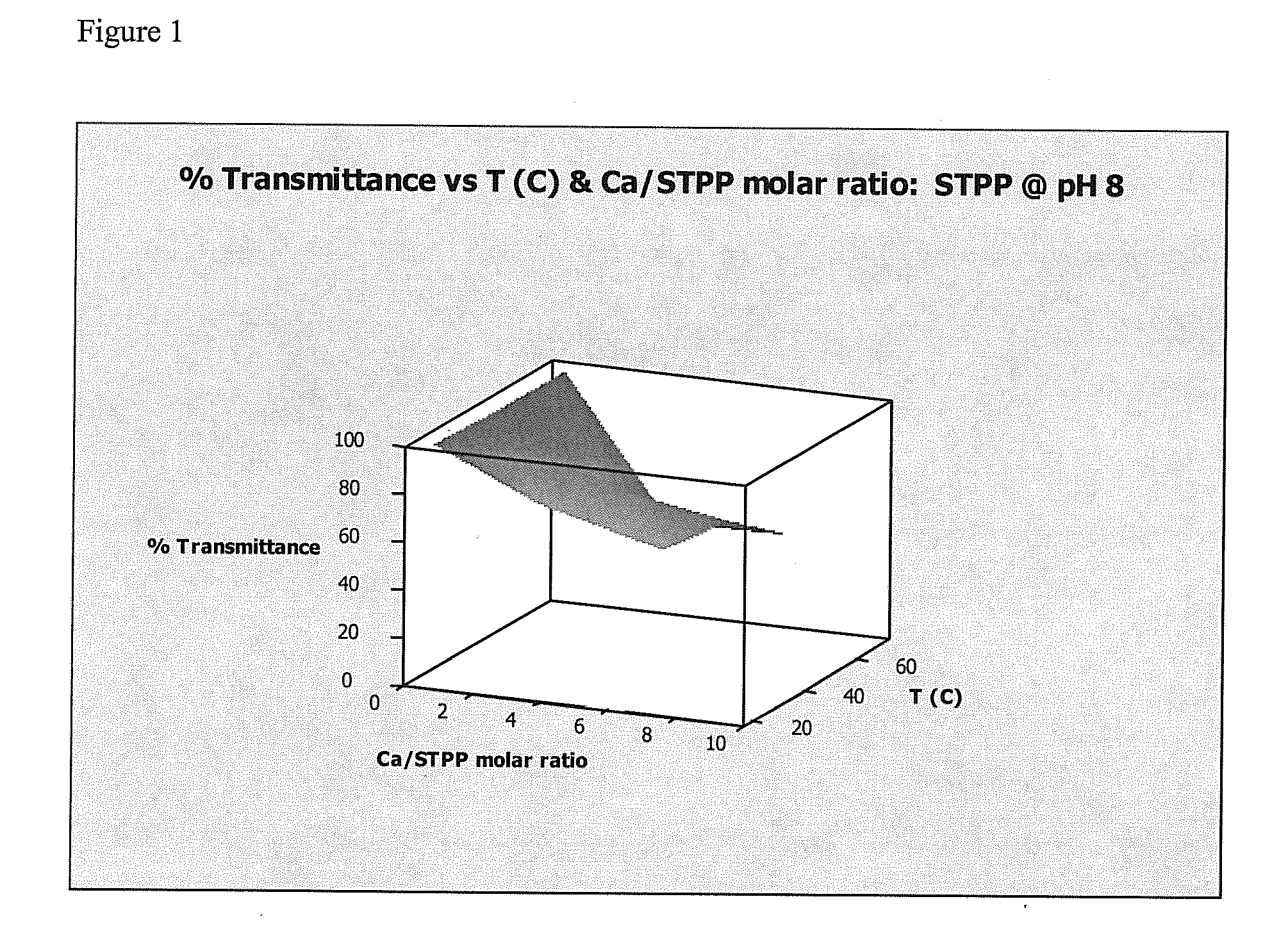
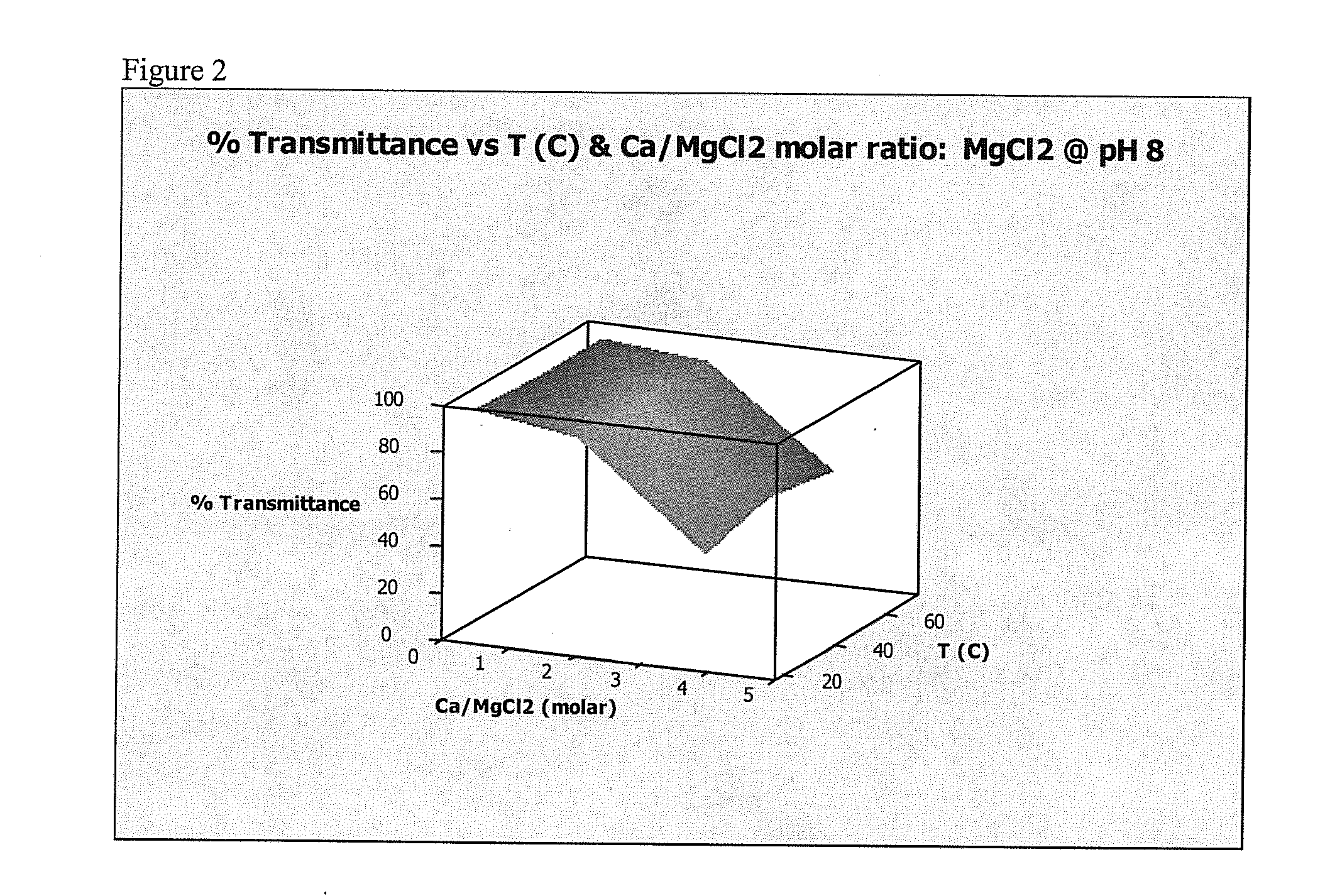
[0105]This Example demonstrates that adding a hardness ion (Mg2+) to water worked as well as a conventional chelating agent or sequestrant (sodium tripolyphosphate (STPP)) at preventing precipitation of calcium salts.
Materials and Methods
[0106]Formation of a precipitate in water reduces the transmission of visible light through the water. A transmittance of 100% indicates that no precipitate formed, while a transmittance of 0% indicates that so much precipitate formed that light no longer passed through the sample. Transmittance was measured for water containing either MgCl2 (present invention) or STPP (comparative example) at pH values of about 8, about 10, and about 12, and at temperatures of about 20° C., about 45° C., and about 70° C. Temperatures were chosen in an attempt to reflect room temperature (20° C.), general laundry temperature (45° C.) and general automatic warewashing temperature (...
example 2
Water Soluble Magnesium Compounds Reduce Formation of Scale from Hard Water
[0117]This Example demonstrates that adding a hardness ion (Mg2+) to water reduced formation of lime scale from hard water.
Materials and Methods
[0118]Tap water with 17 grain hardness and a 2:1 Ca:Mg weight ratio was spiked with various levels of magnesium chloride and then incubated in glass bottles in a 140° F. oven for about two weeks. The bottles were then visually evaluated for lime scale build-up.
Results
[0119]The results are presented in the table below.
Scale onMagnesium Chloride Added (wt-%)Bottle0present0.007present0.067present0.48none2.4none7.2none14none
[0120]The data clearly shows the benefit of adding water soluble magnesium salt to the tap water. In particular, when 0.48 wt-% or more of magnesium chloride was added, no lime scale build-up was observed on the surface of the glass bottle. By contrast, when either no magnesium was present, or when less magnesium chloride was added, lime scale build-up...
example 3
Injecting Water Soluble Magnesium Compounds for Rinse Cycle Reduced Formation of Scale from Hard Water in Warewashing Machine
[0121]This Example demonstrates that, injecting only a hardness ion (Mg2+) into rinse water reduced formation of lime scale from hard water on a dishwasher.
Materials and Methods
[0122]A dishwashing machine was run for 100 cycles using 17 grain hard water for the wash and rinse cycles with no added rinse agent. A dishwashing machine was run for 100 cycles using 17 grain hard water and with water soluble magnesium compound, magnesium sulfate, introduced as the sole rinse agent. The magnesium sulfate was introduced at a molar ratio of magnesium ion to calcium ion of 1:1. No detergent was used in any of the wash cycles.
Results
[0123]FIGS. 7 and 8 are photographs of interiors of dishwashing machines after 100 cycles run using 17 grain hard water only or using 17 grain hard water and with water soluble magnesium compound, magnesium sulfate, introduced as the sole rins...
PUM
| Property | Measurement | Unit |
|---|---|---|
| light transmittance | aaaaa | aaaaa |
| light transmittance | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com