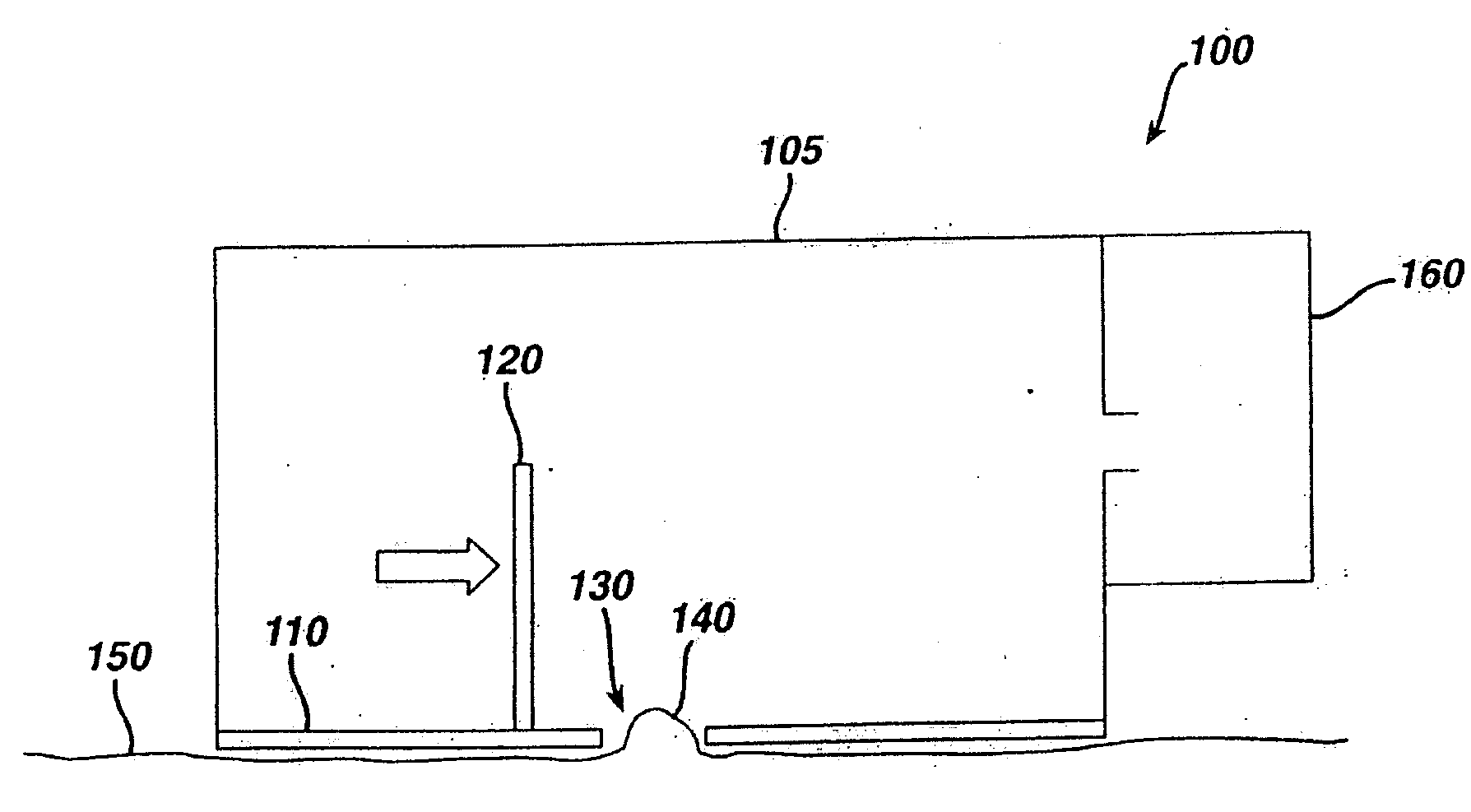
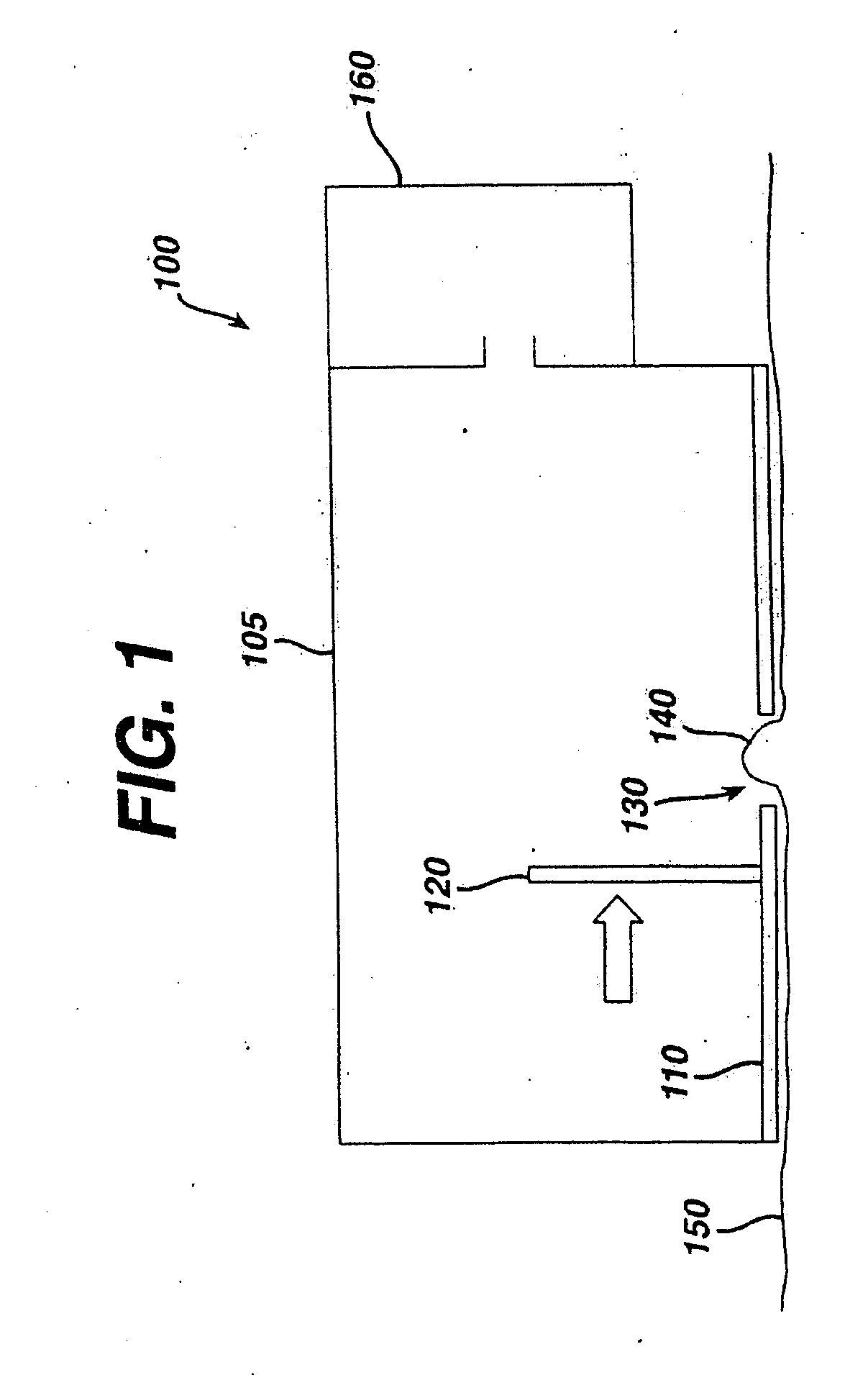
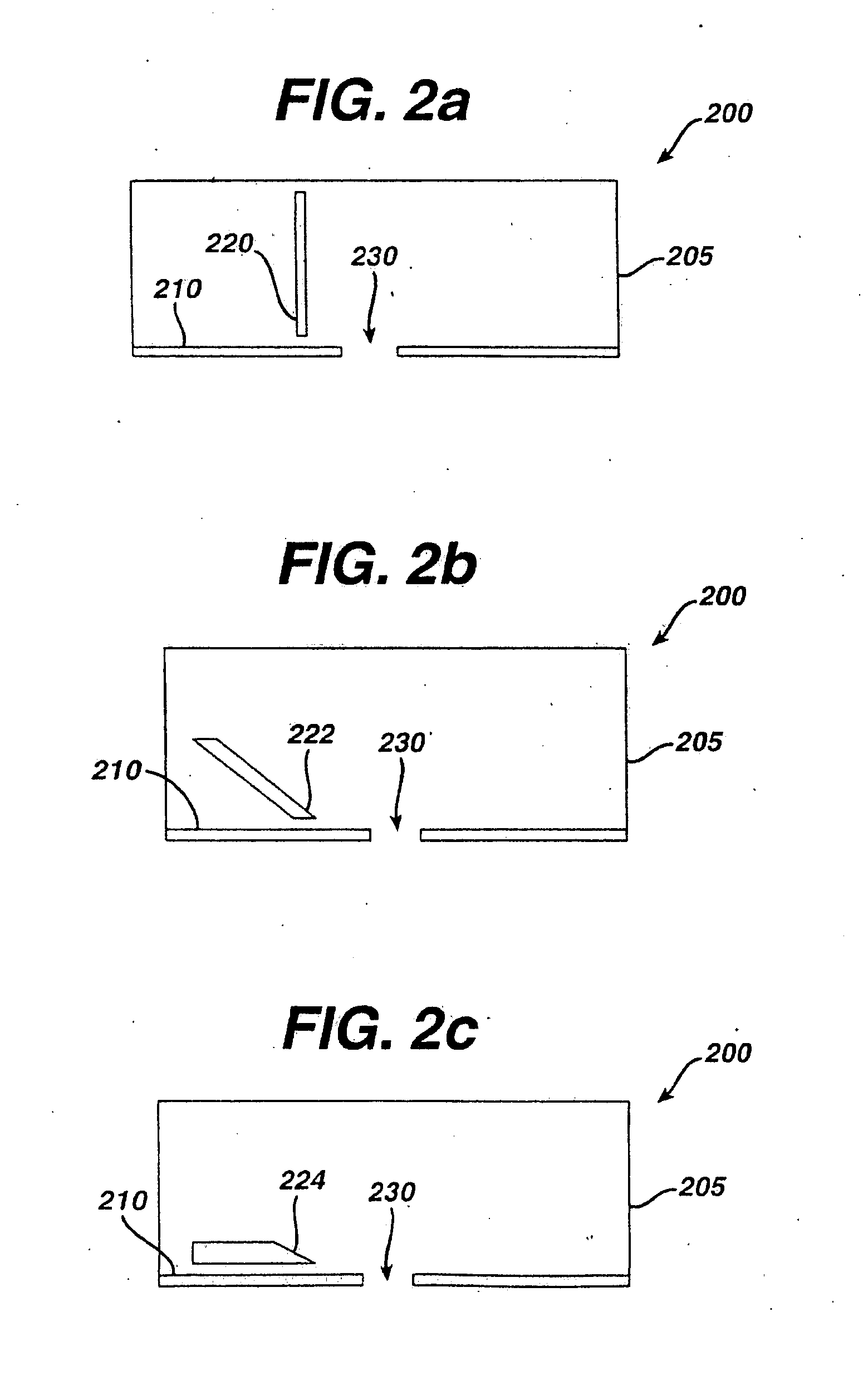
Tissue Ablation by Shear Force for Sampling Biological Fluids and Delivering Active Agents
a technology of biological fluids and tissue ablation, which is applied in the field of tissue ablation by shear force for sampling biological fluids and delivering active agents, can solve the problems of painful lancing, diabetic patients, and painful lancing, and achieve the effect of enhancing sufficient but not excessive membrane ablation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Extraction of Interstitial Fluid (ISF) from Human Skin Following Shear Perforation
[0060]Shear perforation of human skin was performed on the ventral leg (calf) of one male volunteer using a commercially available shaver for facial hairs (National Battery-operated Shaver, ES815B, Mastushita Electric Works, Ltd, Japan). The screen of the shaver was covered with a metallic tape (Ideal Tape Co., Lowell, Mass., USA), so that only 1 cm2 square area at the center of the screen was exposed. The shaver was pressed firmly on the skin for about 5 seconds. Neither pain nor discomfort was experienced by the volunteer. The perforation skin site (0.7 cm2) showed only slight erythema after the skin perforation procedure. A hand-held suction device (MityVac II® vacuum pump, Prism Enterprises, San Antonio, Tex., USA) was applied to the perforation skin site with about 70 cm Hg of suction for a certain time duration. Interstitial fluid (a clear, slightly yellowish liquid) extracted out the pores from ...
example 2
Subcutaneous Injection of Insulin as a Control Experiment for Transdermal Insulin Delivery
[0064]The subcutaneous administration of insulin provides a dose response to which a comparative measure of transdermally delivered insulin can be made. An insulin dose of 5 IU insulin (Regular Iletin® II, Insulin Injection, USP, Purified Pork, Eli Lilly and Company, Indianapolis, Ind., USA) was subcutaneously injected in the rear mid-ventral area of each of four female Yorkshire pigs (weight: 22-26 kg) with a 1 cc Sub-Q Precision Glide® Needle (Becton-Dickinson and Company, Franklin Lakes, N.J., USA). FIG. 5a shows that subcutaneous insulin administration resulted in the expected rapid reduction in blood glucose concentration.
[0065]FIG. 5b shows that a 5 IU insulin subcutaneous injection resulted in a peak insulin concentrations of 31-55 μIU / ml. As the insulin concentrations went back to its basal concentration (FIG. 5b), the blood glucose concentrations also were gradually returned toward its...
example 3
Transdermal Delivery of Insulin into Swine Following Shear Perforation of the Skin
[0066]The stratum corneum is recognized as the primary barrier to percutaneous absorption of drug applied to the skin surface. The densely structured outmost skin layer consisting of flattened and keratinized dead skin cells provides a formidable permeation barrier to most small and virtually all large compounds. It is well known in the field of transdermal drug delivery that a protein drug such as insulin cannot penetrate into the intact skin of human and swine. Our own experiment confirmed this (result not shown). This example investigates in vivo transdermal delivery of insulin into swine following application of shear perforation to remove the stratum corneum barrier at the test site.
[0067]Two female Yorkshire pigs of the same body weight range as those in Example 2 were immobilized with anesthetics. The test site on the side of the chest was prepared by clipping lightly off the hair and cleaning w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| frequencies | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com