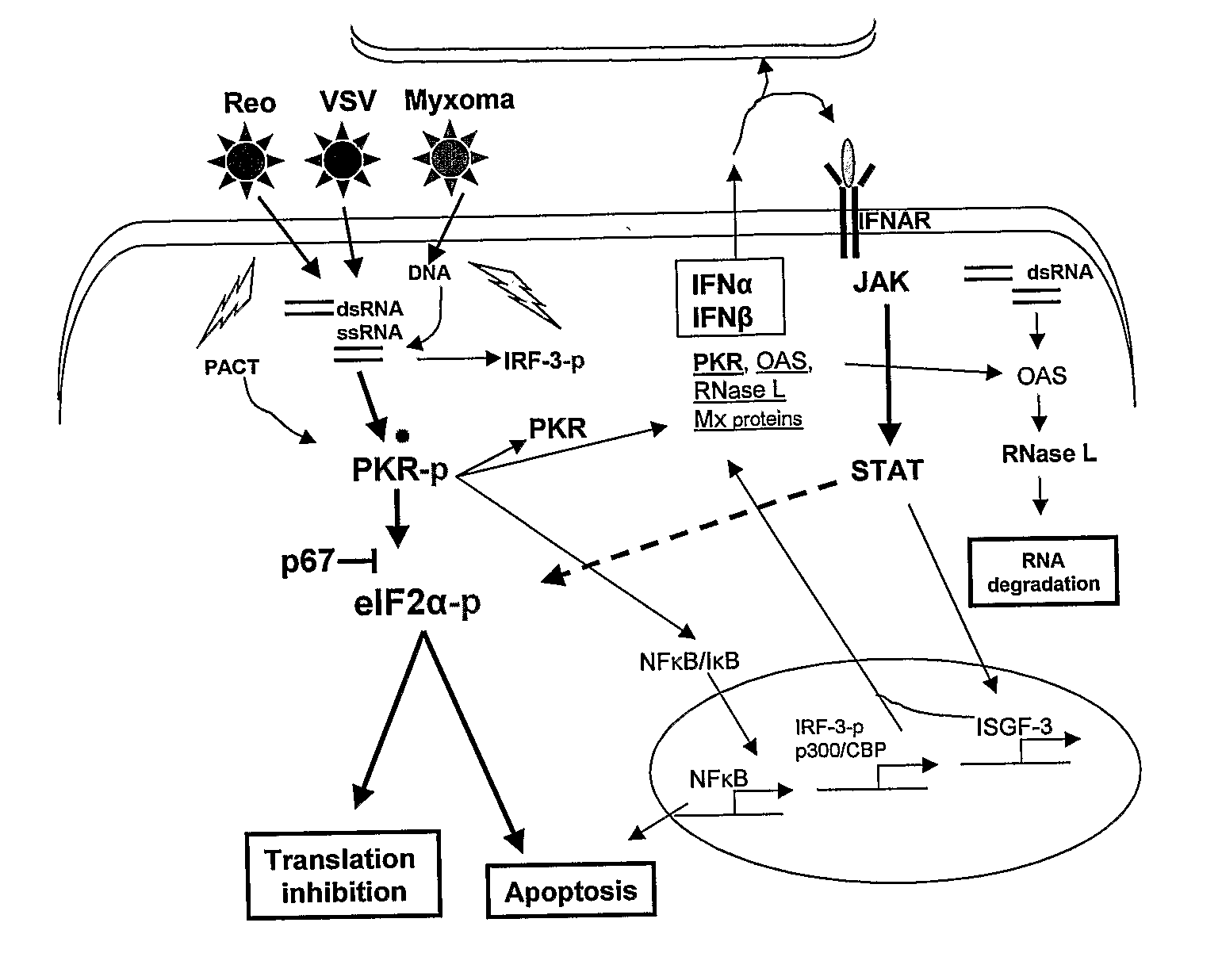
Use of a combination of myxoma virus and rapamycin for therapeutic treatment
a technology which is applied in the field of therapeutic use of myxoma virus and rapamycin, can solve the problems of inability to achieve effective cancer treatment, inability to effectively treat various types of cancer, and inability to work effectively. achieve the effect of improving safety and increasing containmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Infection of Mouse and Human Cell Lines with Myxoma Virus
Virus Strains
[0157]Viral strains used include wildtype MV, MV modified to express either green fluorescence protein (“GFP”) or β-galactosidase (“LacZ”), and killed (“dead”) MV. Viruses were prepped and titred using standard techniques.
Cell Strains
[0158]Mouse experiments were performed using mouse embryo fibroblasts (“MEFs”) derived from a wild-type mouse, and from the following mouse knockouts: IFNα / β receptor homozygous knockout; STAT1 homozygous knockout; PKR heterozygous; RNaseL heterozygous knockout; Mx1 heterozygous knockout; triple PKR / RNaseL / Mx1 homozygous knockout.
[0159]Human experiments were performed on BGMK control cells and human tumour cell lines HT29, HOP92, OVCAR4, OVCAR5, SK-MEL3, SK-MEL28, M14, SKOV3, PC3, DU145, CAKI-1, 786-0, T47D, MDAMB 435, SF04, U87, A172, U373, Daoy and D384 as described in Stojdl et al., Cancer Cell (2003) 4: 263-275.
Methods
[0160]Generally, assays and experiments were performed as descr...
example 2
Effect of Rapamycin on the Kinetics of Myxoma Virus Replication in Restrictive Cell Lines
Virus Strains
[0180]Viral strains used include wildtype MV (“vMyxLac”), and MV modified to have the MT-5 gene knocked out (“vMyxLacT5-”). Viruses were prepped and titred using standard techniques.
Cell Strains
[0181]Human experiments were performed on BGMK primate control cells, RK-13 rabbit control cells and normal human fibroblasts A9, restrictive human tumour cell lines 786-0 (renal), ACHN (renal), HCT116 (colon), MCF-7 (breast), MDA-MB-435 (breast), M14 (melanoma) and COL0205 (colon).
Methods
[0182]Generally, assays and experiments were performed as described in Lalani et al. Virology (1999) 256: 233-245; Johnston et al. J Virology (2003) 77(13): 7682-7688; and Sypula et al. Gen Ther Mol Biol (2004) 8: 103.
[0183]For viral growth curves, cells were grown in vitro in a monolayer, and pretreated with 20 nM rapamycin or a control (1:5000 dilution of DMSO) prior to infection with virus.
[0184]Samples o...
example 3
Molecular Consequences of Inhibiting mTOR in the Context of Myxoma Virus Infection
[0205]Western Blot analysis (FIG. 43) was performed using cell lysates from 786-0 cells, a Type II cancer cell line where rapamycin enhances myxoma virus infection. Lysates were collected 16 hours post infection with either vMyxLac or vMyxT5KO at an MOI of 3, or without virus infection. Indicated lanes contain protein from cells that were pretreated with 20 nM rapamycin (designated R) or appropriate vehicle control (1:5000 dilution of DMSO, designated D) for 6 hours before infection. The blots were probed using primary antibodies directed against the indicated proteins.
[0206]As demonstrated, myxoma virus infection affects many of the signaling pathways that converge on mTOR, the physiologic target of rapamycin. In the context of infection with either wild type (vMyxLac) or MT-5 deficient (vMyxT5KO) virus, where rapamycin has a beneficial effect on virus replication, global effects are observed in many ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com