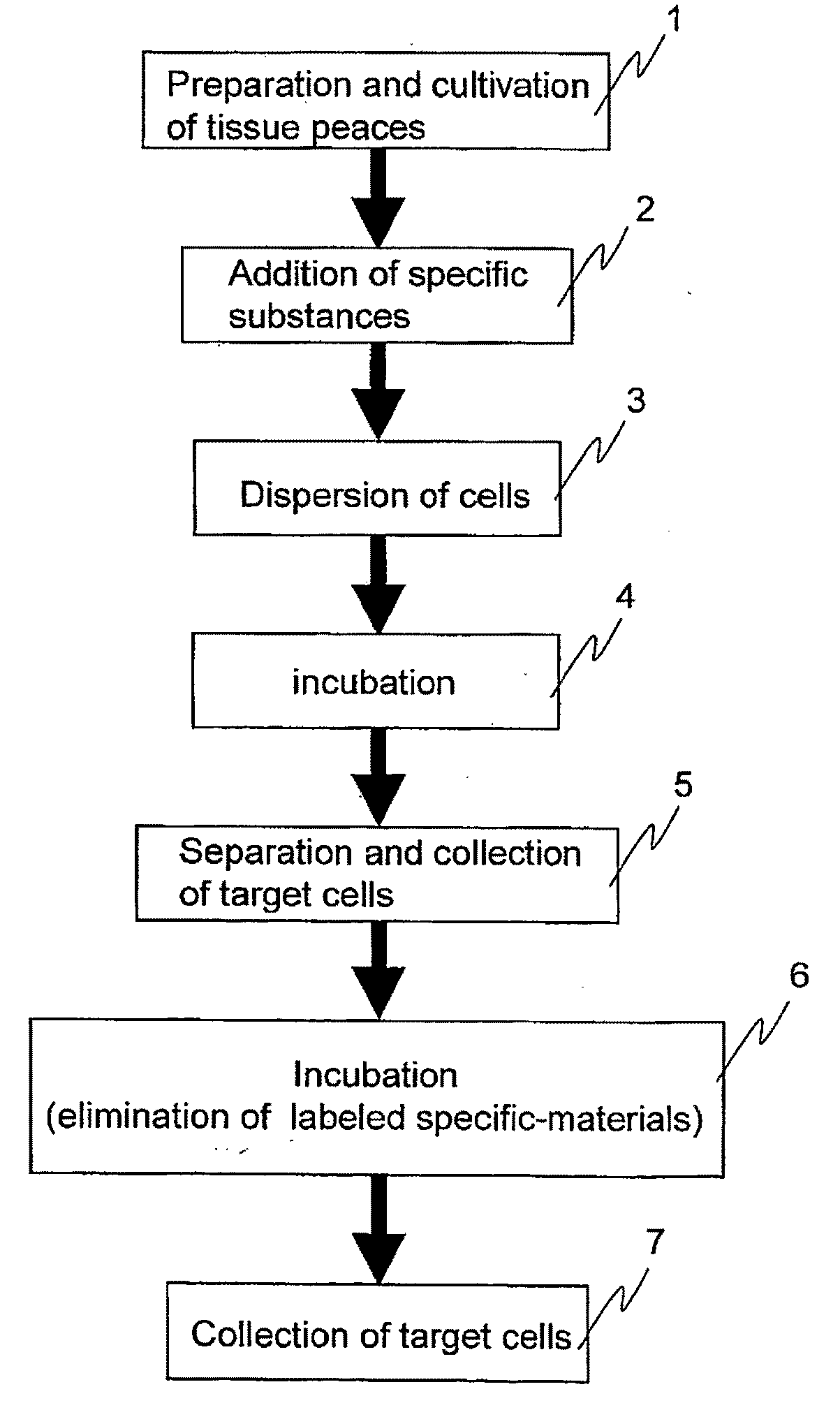
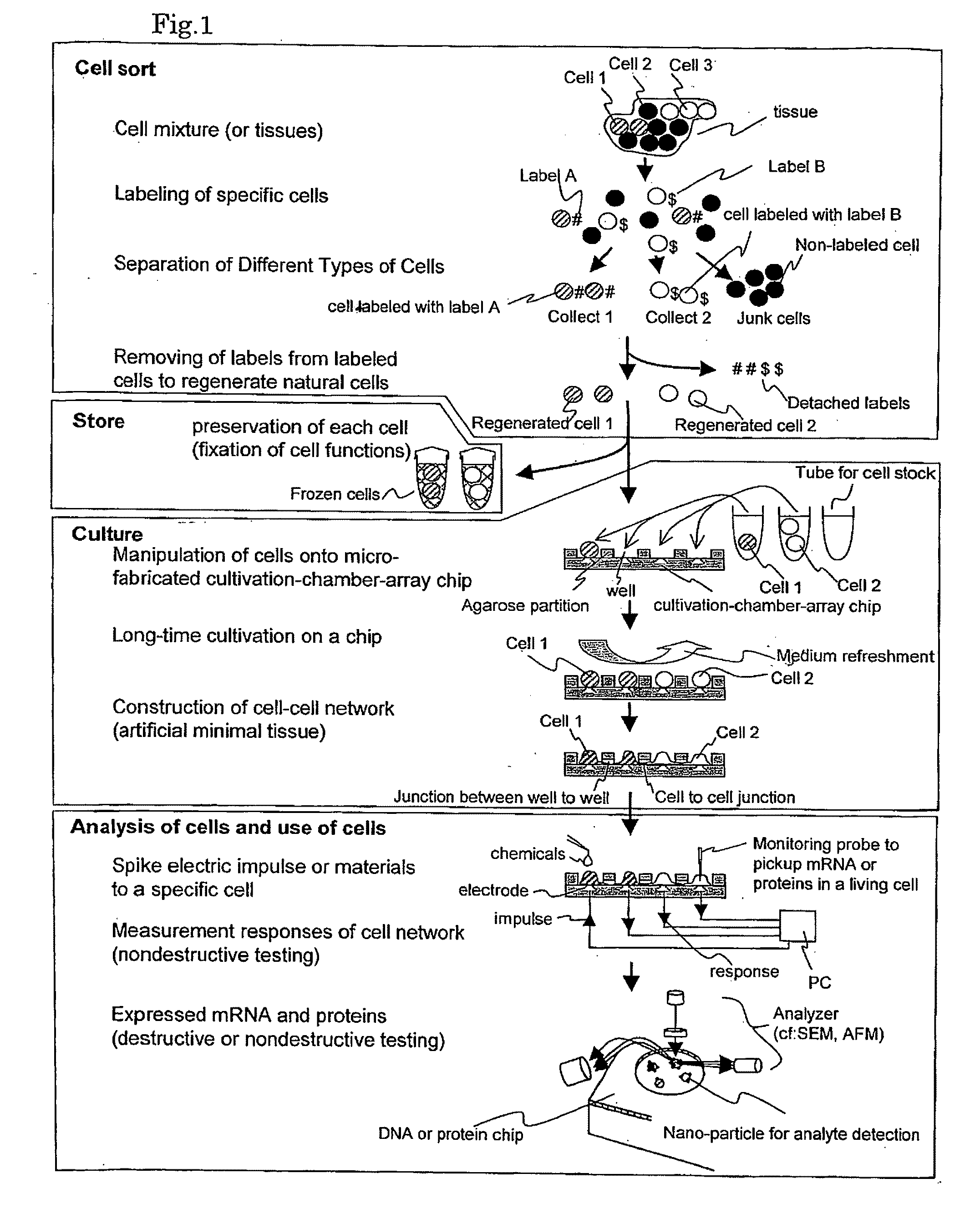
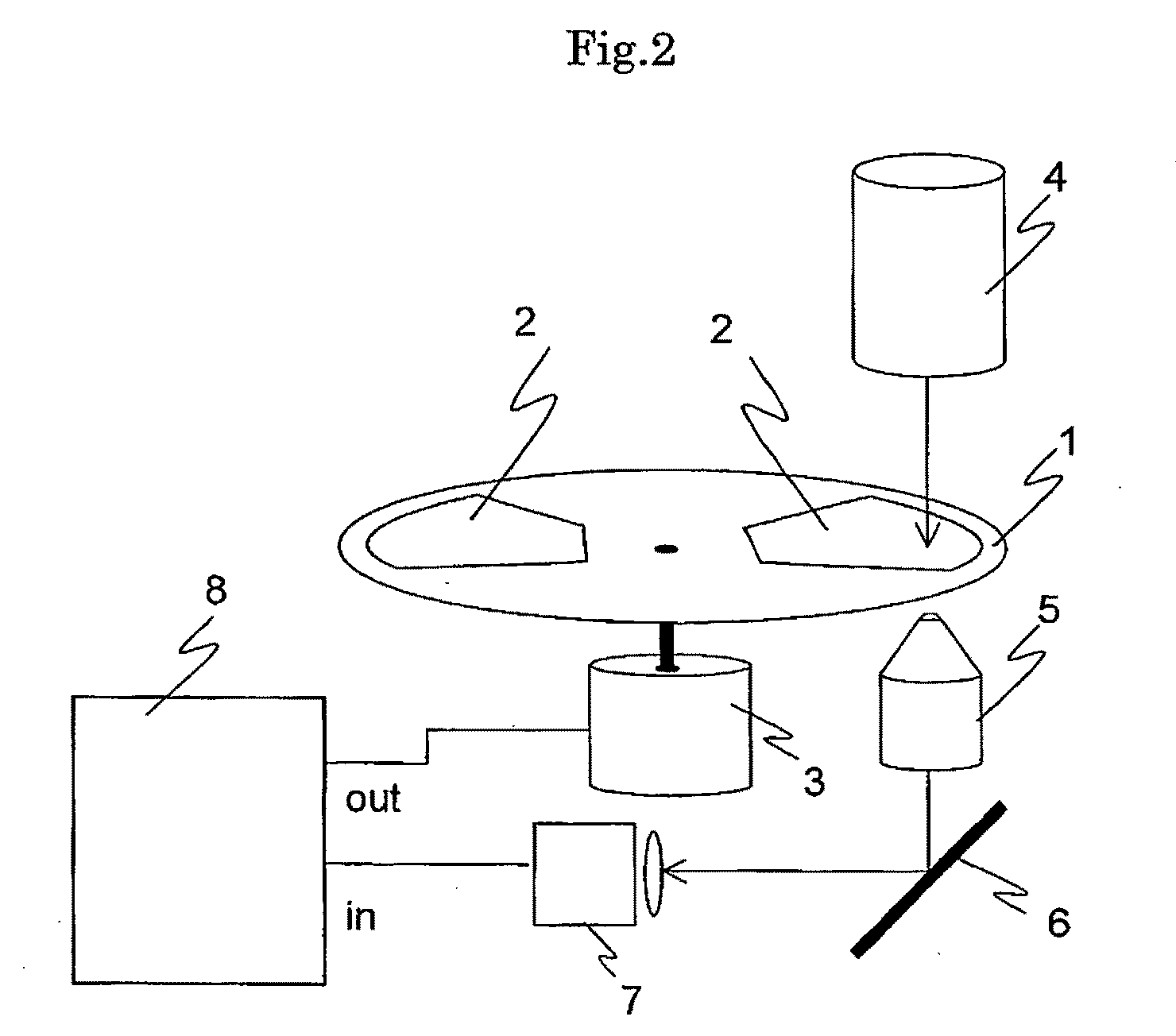
Cellomics system
a cell and system technology, applied in the field of cellomics, can solve the problems of insufficient clarification of the relationship between sensitivity and reproducibility, inability to obtain a sufficient quantity of amplification products, and inability to perform amplifying operations. to achieve the effect of low cost and high speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0285]An example is described hereinafter with identification element made up of RNA aptamer as a labeling substance to label cell surface antigen CD4 and β-phycoerythrin as a marker substance in isolating and collecting cells bonded with the RNA aptamer. That is, cells presenting the cell surface antigen CD4 is specifically labeled with the β-phycoerythrin-modified RNA aptamer as described hereinabove, and then a cell isolation chip, which is a cell sorter formed on a plastic chip substrate as disclosed in Japan Patent Application 2004-379327.
[0286]FIG. 17 is a view showing a process flow of specifically labeling a cell presenting the cell surface antigen CD4 with the β-phycobiliprotein-modified RNA aptamer and thereafter isolating the cell with a cell sorter. The top-most part of FIG. 17 shows a sample 10 with two kinds of cells 3 and 4 in a mixed manner. The cells 3 present a cell surface antigen CD4 indicated by a triangular marked out in black with a reference numeral 1. The ce...
example 6
[0350]In the examples as described above, a pipet is described in each case as it has one function, while in Example 6, an example of a pipet having two functions is described.
[0351]FIG. 27(a) is a view showing a tip of a pipet 81 having two flow paths separated by a partition plate 82, and FIG. 27(b) is a view showing configuration in which a pipet 89 is provided inside a pipet 87 to form two flow paths.
[0352]In the configuration shown in FIG. 27 (a), by making a first flow path 83 sufficiently larger than a second flow path 84, and by designing a structure in which cell suspension can be supplied from the first flow path 83 and dilution can be supplied from the second flow path 84, the pipet 11 and the pipet 20 described in Example 2 can be integrated. It is needless to say that controls of the respective flow paths are carried out with respective independent syringe pumps.
[0353]In the configuration shown in FIG. 27(b), the inner pipet 89 has an inner diameter of 50 μm, and spacin...
example 5
[0834]In order to make the twenty-first embodiment of the present invention more effective, it is preferable to eliminate a charge of probe itself and introduce a large amount of minus charge to the free terminal of the probe. For instance, PNA (Peptide Nucleic Acid) in which a phosphodiester bond of synthetic oligonucleotide is changed to a peptide bond, or CAS (Cysteine Antiesnse Compound) which includes S-carboxydimethyl-L-cysteine as a base frame may be used as a non-charged probe.
[0835]Since main chains of the polymer PNA and CAS have no charge, an electrostatic repelling force does not work with the target polynucleotide. As they have amino group and carboxyl group on the terminals thereof respectively, when an amino group is used at the fixed terminal, the fee terminal is naturally changed to a negatively charged carboxyl group. Further it is possible to make the probe act quickly in response to the electrode on the surface of the substrate by introducing a large amount of mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| covalent | aaaaa | aaaaa |
| fluorescent | aaaaa | aaaaa |
| fluorescent detection | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com