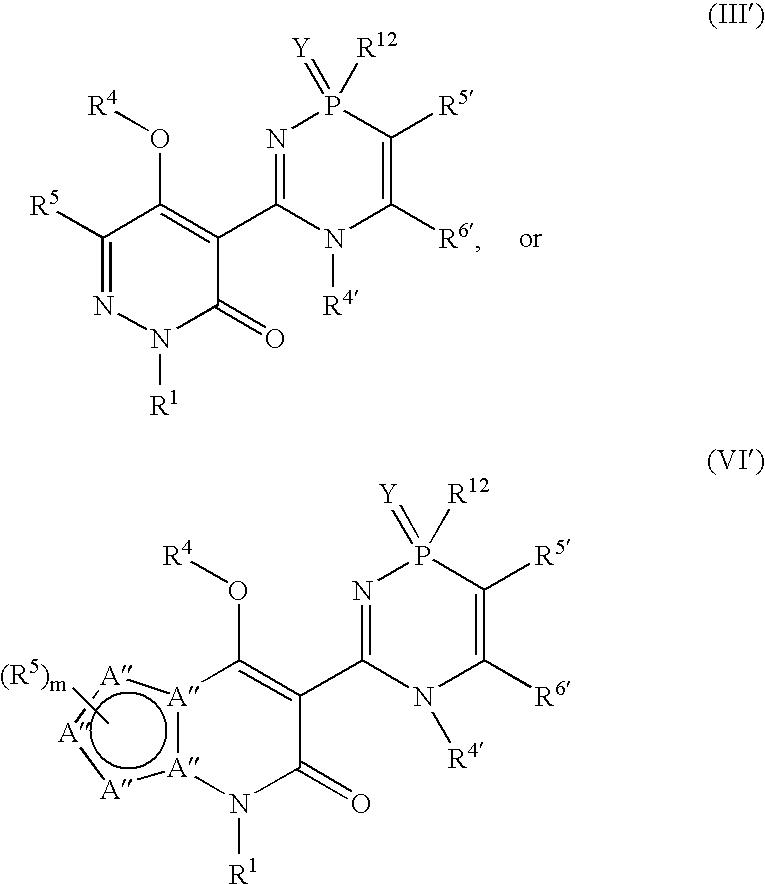
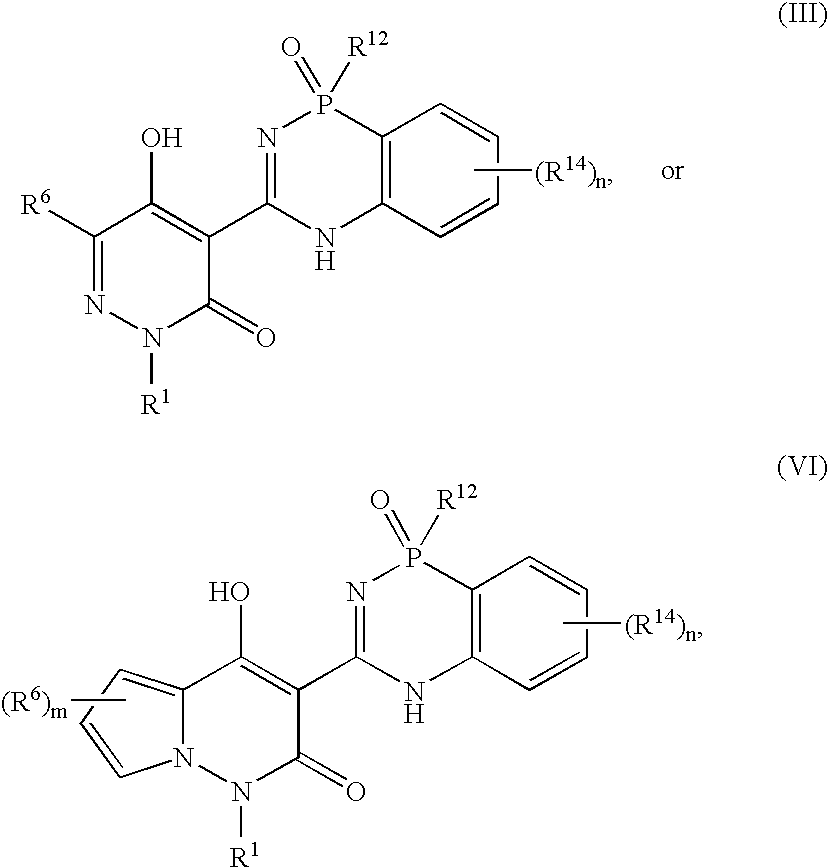
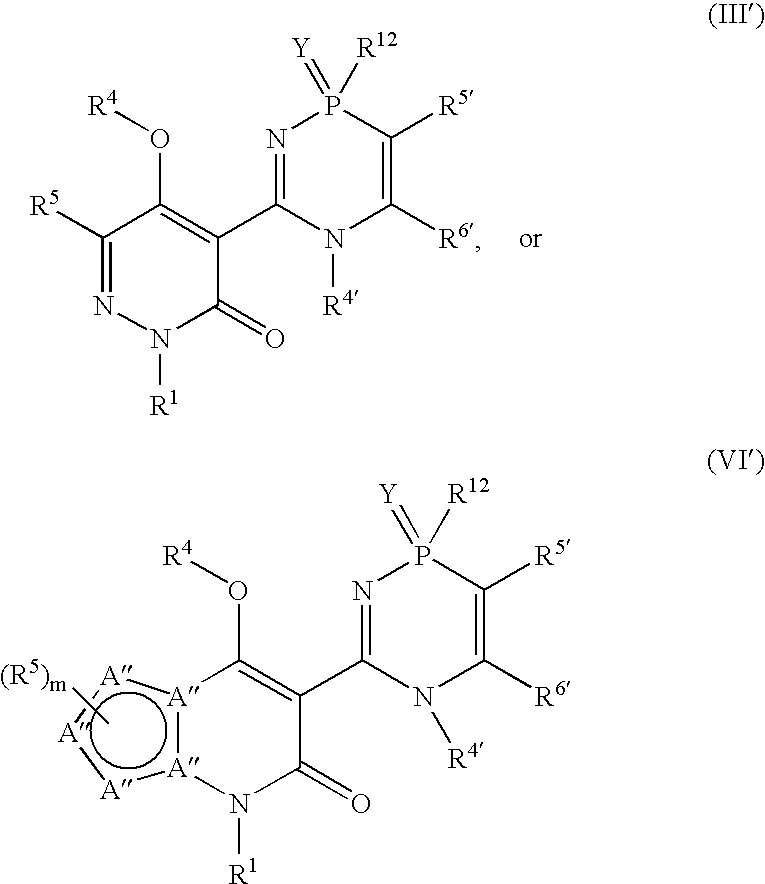
Phosphadiazine hcv polymerase inhibitors iii and vi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0344]As used herein, the symbols and conventions used in these processes, schemes and examples, regardless of whether a particular abbreviation is specifically defined, are consistent with those used in the contemporary scientific literature, for example, the Journal of the American Chemical Society or the Journal of Biological Chemistry. Specifically, but without limitation, the following abbreviations may be used in the examples and throughout the specification: g (grams); mg (milligrams); mL (milliliters); μL (microliters); mM (millimolar); μM (micromolar); Hz (Hertz); MHz (megahertz); mmol (millimoles); hr (hours); min (minutes); TLC (thin layer chromatography); HPLC (high performance liquid chromatography); SCX (strong cation exchange); MS (mass spectrometry); ESI (electrospray ionization); Rt (retention time); SiO2 (silica); THF (tetrahydrofuran); CD3OD (deuterated methanol); CDCl3 (deuterated chloroform); DCE (dichloroethane); DCM (dichloromethane); DMF (dimethyformamide); D...
example 41
1-(1-ethoxy-1-oxo-4H-7-methanesulfonaminyl-benzo[1,2,4]phosphadiazine-3-yl)-1-N-(3,3-dimethylbutyl)-4-hydroxy-3-thiophenyl-pyridazin-6-one
[0486]
[0487]Example 41 was synthesized from intermediate 26 and intermediate 71. To a solution of intermediate 71 (0.140 mmol) in dioxane (2 ml) was added intermediate 26 (0.210 mmol). This mixture was treated dropwise with trimethyl aluminum (0.840 mmol) and stirred in a sealed tube at 80° C. for 4 hours. After, the reaction mixture was quenched with HCl 1N, diluted with TBDME or ethyl acetate, washed with water and brine, dried over sodium sulfate and concentrated in vacuo after purification with preparative HPLC to give example 41, which was a white lyophilized powder. Example 41 was characterized by the following spectroscopic data: 1H NMR (DMSO-d6, 400 MHz) δ (ppm) 0.96 (s, 9H), 1.15-1.18 (t, J=6.95 Hz, 3H), 1.58-1.62 (t, J=7.94 Hz, 2H), 3.04 (s, 3H), 3.94-3.90 (m, 2H), 3.99-4.01 (m, 2H), 7.47-7.52 (m, 4H), 7.58 (s, 1H), 8.46 (s, 1H), 10.15 (...
example 42
1-1-hydroxy-1-oxo-4H-7-methanesulfonaminyl-benzo[1,2,4]phosphadiazine-3-yl)-1-N-(3,3-dimethylbutyl)-4-hydroxy-3-thiophenyl-pyridazin-6-one
[0488]
[0489]Example 42 was synthesized from example 41. Example 41 (1 eq) and trimethylsilylbromide (10 eq) dissolved in 1,2-dichloroethane (10 ml), were stirred in sealed tube, at 60° C., for 2 hours. The reaction mixture was then evaporated, diluted in methanol (1 ml) and added to HCl 1N. (15 ml). The precipitate was filtered, washed with water and dried over P2O5 to give example 42, which was a white lyophilized powder. Example 42 was characterized by the following spectroscopic data; and MS (ESI, EI+) m / z=552 (MH+). Example 42 is equivalent to Compound III-21.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com