Asthma associated factors as targets for treating atopic allergies including asthma and related disorders
a technology of asthma and associated factors, which is applied in the field of modulating activities associated with the il9 pathway for the treatment of atopic allergies and related disorders, can solve the problems of allergic inflammation, huge burden on health-care resources, damage to surrounding tissues, etc., and achieves the effects of increasing calcium-dependent chloride secretion, enhancing th2-associated responses, and increasing chloride secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
cDNA Difference Analysis of IL-9 Expressed Genes
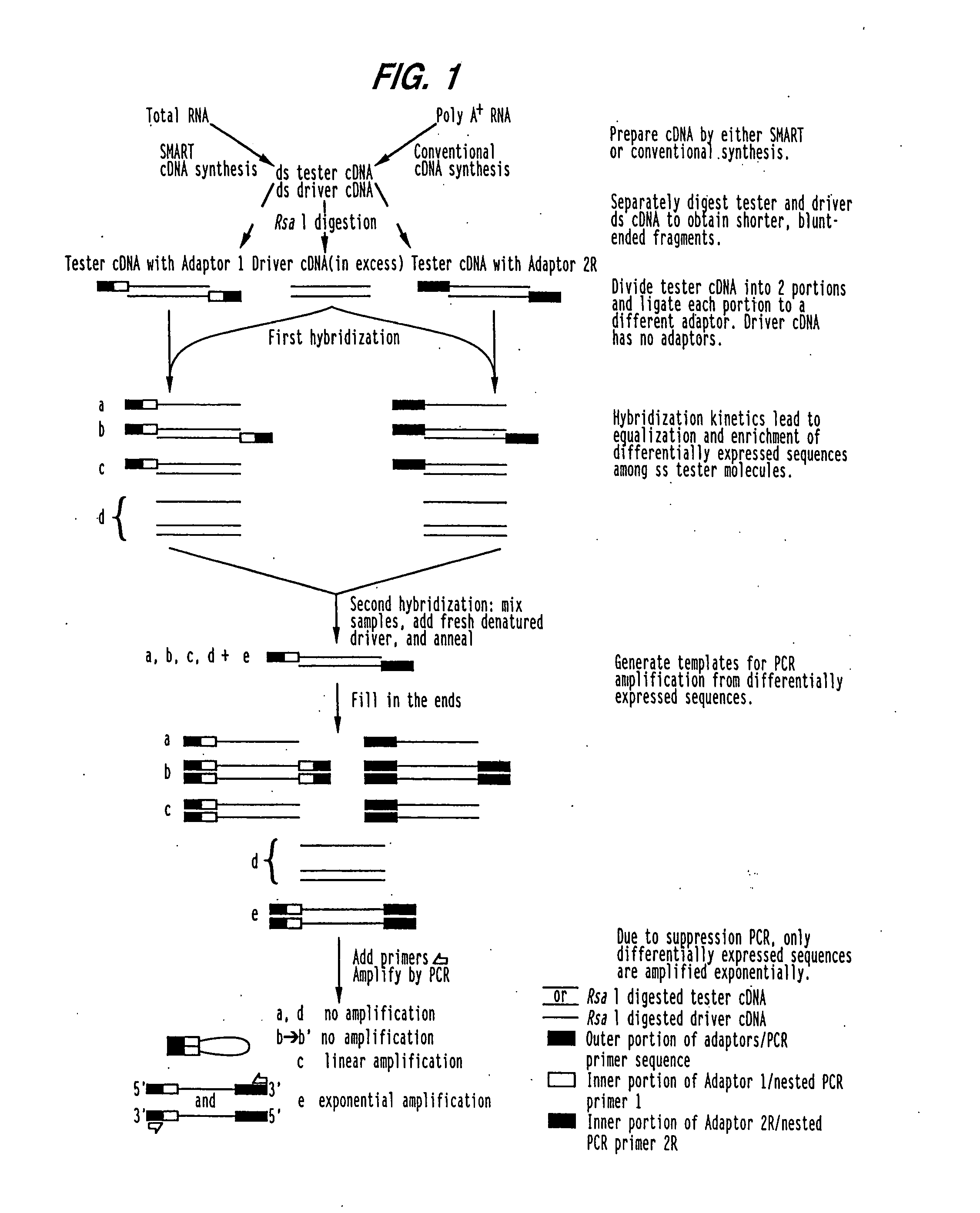
[0138]Lungs extracted from transgenic IL-9 mice (Tg5) were used to isolate IL-9 induced genes. Tg5 is a FVB mouse overexpressing the IL-9 gene as previously described (Renauld et al., 1994). This strain has been shown to overexpress IL-9 in most tissues of the mouse. In order to identify specific IL-9 induced genes, suppressive PCR cDNA difference analysis was performed on mRNA from lungs of Tg5 mice and parental FVB mice using a commercially available PCR-select cDNA subtraction kit (Clonetech).
[0139]cDNA synthesis. Total RNA was prepared from lungs of FVB and Tg5 mice using Trizol as described by the manufacturer (Gibco / BRL). Lungs were removed from euthanized mice and frozen in liquid nitrogen. Frozen lungs were then placed in Trizol and pulverized using a tissue grinder. Polyadenylated RNA was purified from total RNA with oligo(dT) cellulose columns (Pharmacia). Double stranded cDNA was prepared using Superscript II reverse transcr...
example 2
Identification of the Murine ICACC-1 cDNA in the Lung of IL-9 Transgenic Mice
[0141]ICACC-1 probes as described in Example 1 were used to probe a murine lung cDNA library (Clonetech) according to the manufacturers recommendation. One million recombinant clones were screened and several overlapping phage were identified. Subsequent screens enabled identification and isolation of a single plaque containing a phagemid which was then transformed into a double-stranded plasmid by phage rescue according to the manufacturers protocol. Recombinant clones were prepared and sequenced using primers directed to the plasmid vector as well as internal sequences identified from the partially subtracted probe. Clones were then aligned and contiged to generate the full-length sequence.
[0142]The 2931 bp cDNA isolated contained an open reading frame encoding a protein of 925 amino acids. FIG. 2 shows the nucleotide and amino acid sequence of the murine ICACC-1 cDNA. A nucleotide BLAST (Altschul et al.,...
example 3
ICACC-1 is Induced in vivo by IL-9 in Murine Cells
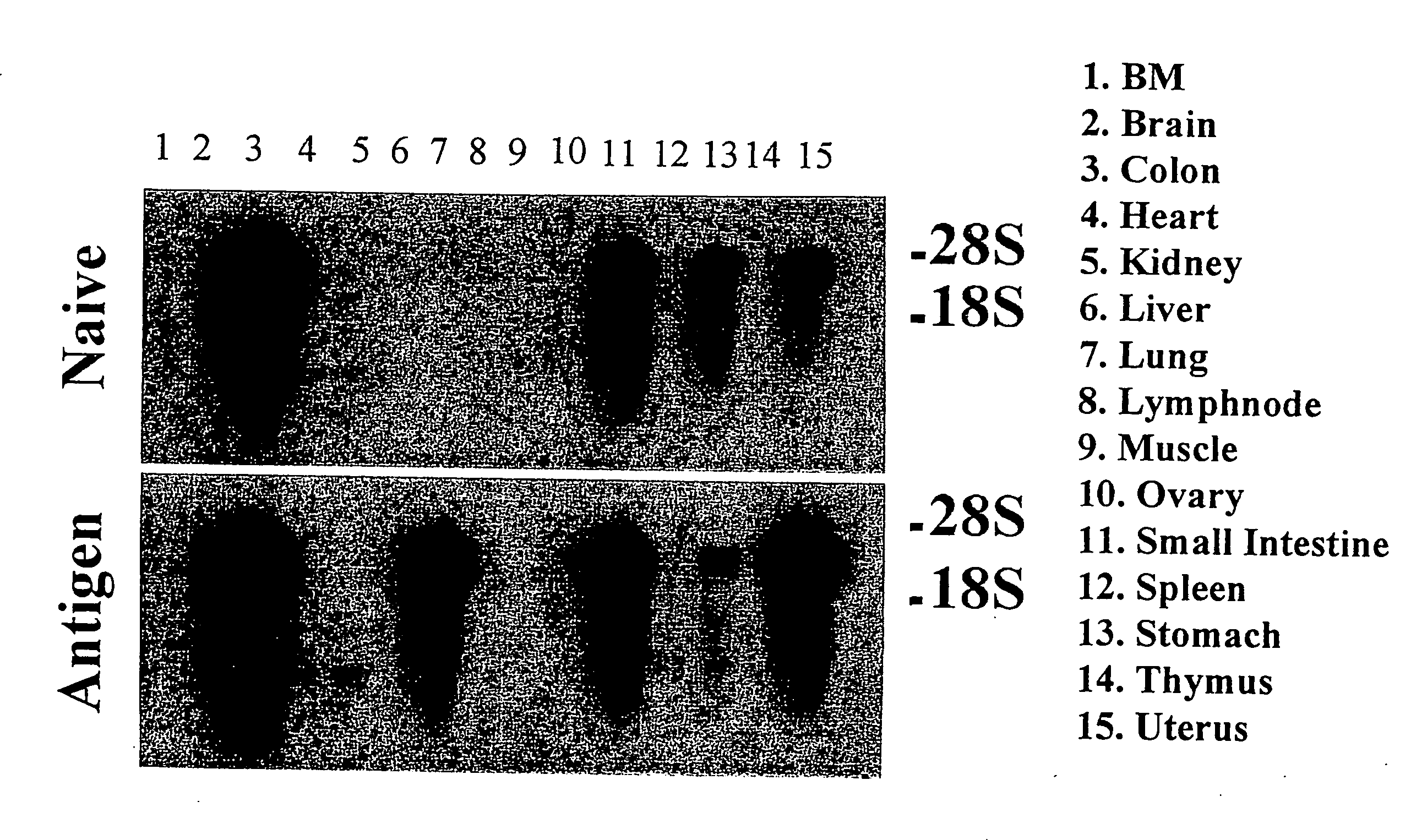
[0143]To confirm that ICACC-1 is induced by IL-9 in the lung, RNA from the lungs of Tg5 and FVB mice were isolated as described in Example 1. cDNA was generated using random hexamers (Pharmacia) and Superscript II (Gibco / BRL) as suggested by the manufacturer. Message was analyzed by PCR as described (Nicolaides et al., 1995) and via Northern blot. Primers used to generate murine ICACC-1 message were; sense 5′-CCAGATCCACACCAAAACGAGAAG-3′ (SEQ ID NO:7) (nucleotides 689-712) and antisense 5′-CACTGTCAAAGGTCACCATCCCGA-3′ (SEQ ID NO:8) (nucleotides 1041-1064) which produce a gene product of 376 bp. DHFR was assayed as an internal control to measure for cDNA integrity using primers previously described Nicolaides et al., 1991). Amplification conditions used were 95° C. for 30 seconds, 58° C. for 1.5 minutes and 72° C. for 1.5 minutes for 35 cycles. For Northern blot analysis, total RNA derived from Tg5 or FVB lungs was electrophoresed on 1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com