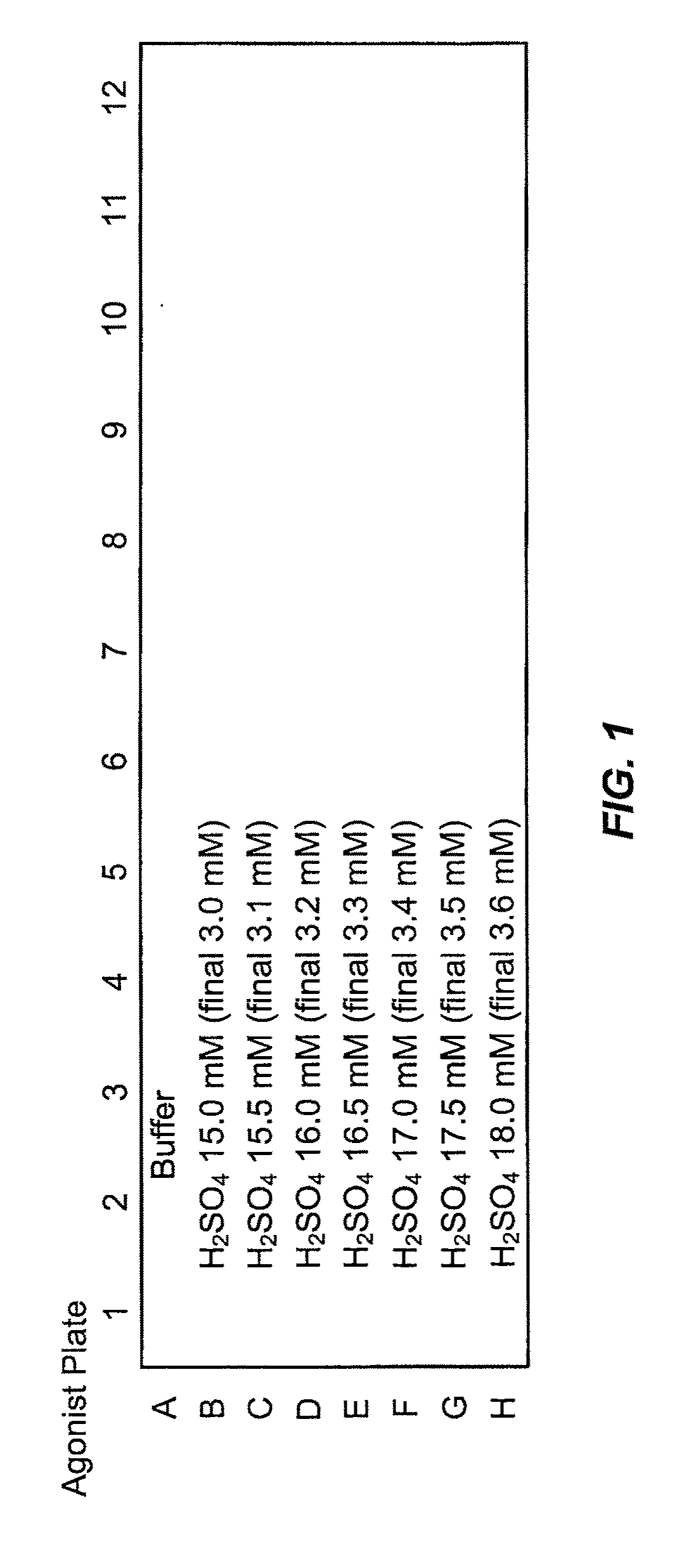
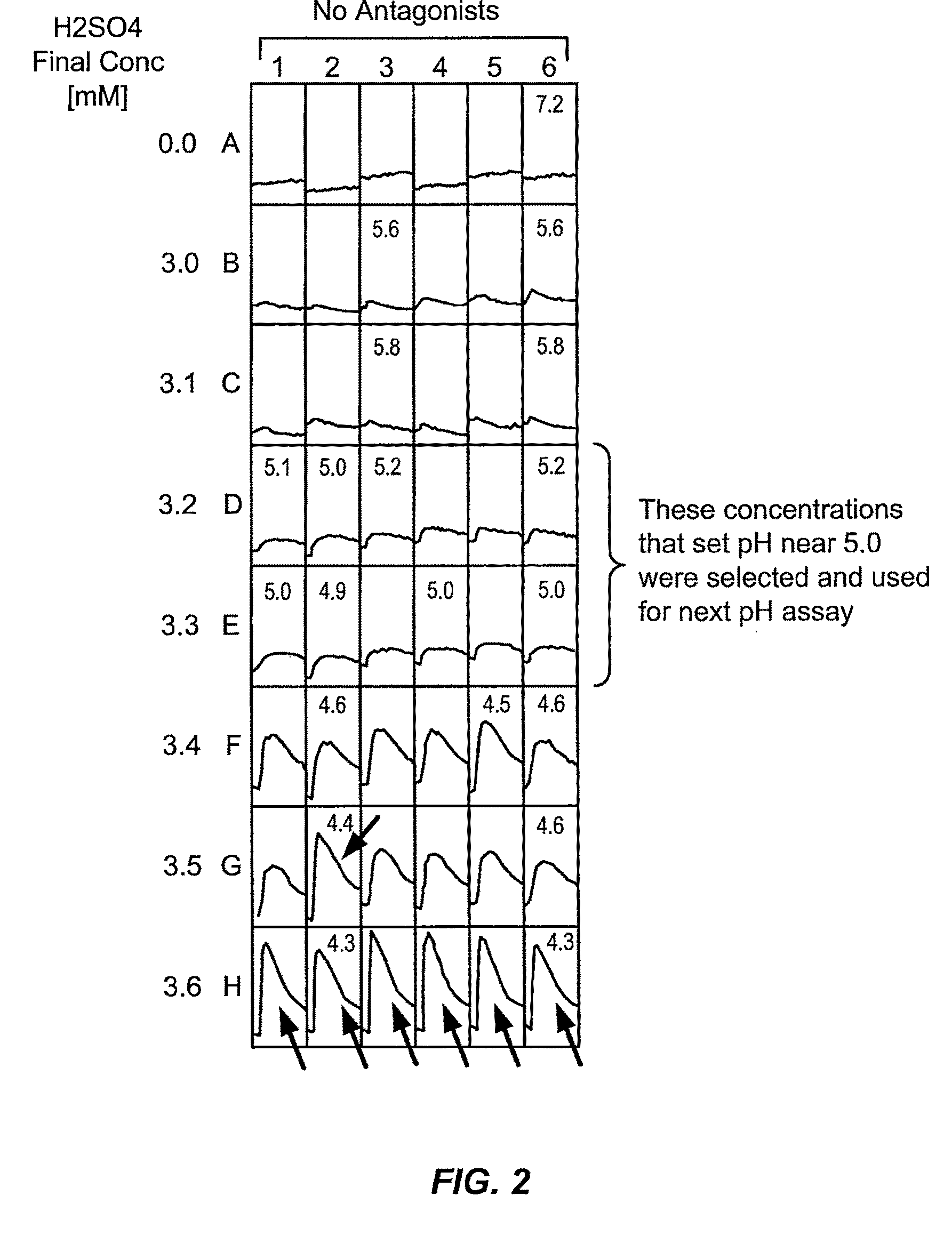
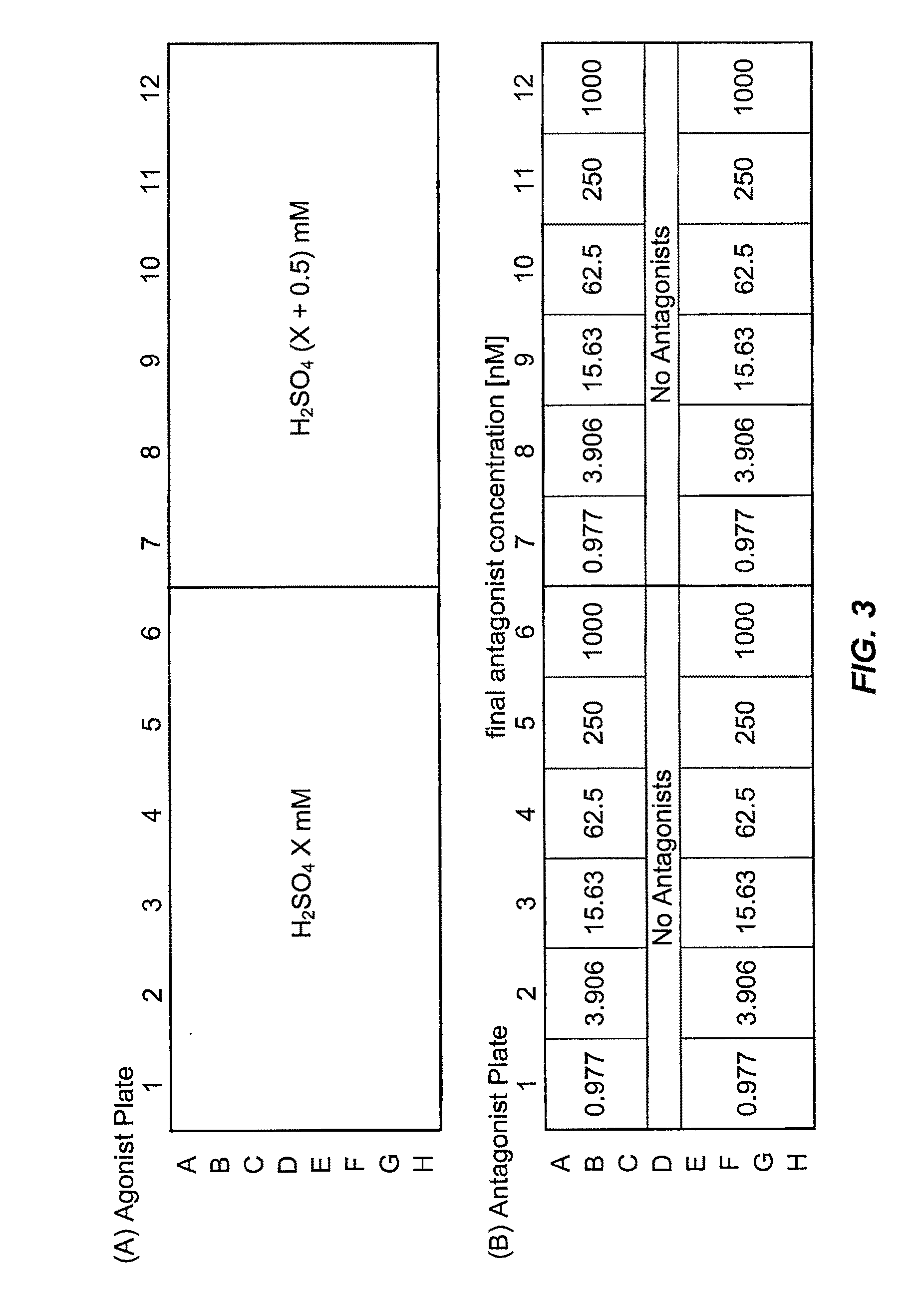
Trpv1 antagonists including sulfonamide substituent and uses thereof
a technology of sulfonamide and antagonist, which is applied in the field of compound formula i, can solve the problems of nausea, headache, weakness, and no treatment of existing ui patients with significant adverse side effects, and achieve the effect of reducing the risk of ui
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Syntheses of Compounds Z1, J1, D2, S1, I6, Y1, J6
2,3-Dichloro-5-formylpyridine
[1303]
[1304]To a 500 mL round-bottom flask, manganese oxide (43.5 g, 0.50 mol) was added to a solution of 2,3-dichloro-5-hydroxylmethylpyridine (64, 8.10 g, 50.0 mmol) in anhydrous CH2Cl2 (150 mL). The reaction mixture was stirred at a temperature of about 25° C. for 48 h, filtered through CELITE, and concentrated under reduced pressure. The mixture was chromatographed by a silica gel chromatography column eluting with a gradient of ethyl acetate (0%-40%) / hexanes to provide 7.2 g of 65 (90% yield). 1H NMR (400 MHz, CDCl3) δ 10.08 (1H, s), 8.77 (1H, d, J=1.97 Hz), 8.25 (1H, d, J=1.97 Hz). LC / MS (M+1): 176.
2,3-Dichloro-5-vinylpyridine
[1305]
[1306]To a stirred slurry of methyltriphenylphosphonium bromide (10.0 g) in toluene (200 mL) at 0° C. was added potassium t-butoxide (3.07 g) portionwise to produce a yellow slurry. After 1 hr, the reaction mixture was cooled to −20° C. and 65 (4.0 grams, 22.72 mmol) d...
example 2
The Synthesis of Compound N1
2-bromo-3,5-dichloropyridine
[1328]
[1329]A 100 mL round-bottom flask equipped with a condenser was charged with 1.82 g of compound 71 (10.0 mmol) and propionitrile (20 mL), 3.06 g TMSBr (20.0 mmol) was slowly added to the above solution. The reaction mixture was stirred at 100° C. under nitrogen for 14 hrs, then cooled to a temperature of about 25° C. and diluted with EtOAc (100 mL). The EtOAc layer was isolated, dried, and concentrated under reduced pressure to provide 72 as a yellowish solid (>99% yield).
tert-butyl 4-(3,5-dichloropyridin-2-yl)-4-hydroxypiperidine-1-carboxylate
[1330]
[1331]Under nitrogen atmosphere, to a 200 mL diethyl ether solution of 72 (2.27 g, 10 mmol) at −78° C. was dropwise added an ice-cold 1.7M t-BuLi in pentane solution (6 mL, 10.5 mmol) via a syringe while maintaining the mixture below −75° C. After completion of the addition, the reaction mixture was stirred at −78° C. for 2 hrs. Then 20 mL of an anhydrous diethyl ether solutio...
example 3
Syntheses of Piperazine Compounds K6, L6, M6, V6 and W6
2,3-dichloro-5-vinylpyridine
[1342]
[1343]To a suspension of methyltriphenylphosphonium bromide (PPh3CH3Br, 7.08 g, 19.8 mmol, Sigma-Aldrich) in THF (40 mL) at 0° C. was added dropwise a 0.5N solution of potassium bis(trimethylsilyl)amide [K(N(TMS)2)] in toluene (39.6 mL, 19.8 mmol, Sigma-Aldrich). Then the resultant mixture was stirred at 0° C. for 1 hour. To the mixture was added a solution of 65 (3.17 g, 18.0 mmol) in THF (20 mL) at 0° C. The reaction mixture was stirred for 2 h at 0° C. The reaction was quenched with water, and the mixture was extracted three times with EtOAc (150 mL for each extraction). The organic portions were combined, washed with brine, and concentrated to dryness. Compound 66 was obtained as a slight yellowish oil via flash chromatography using ethyl acetate / hexane gradient as an eluent (64% yield). 1H NMR: (CDCl3) δ 8.28 (d, J=2.1 Hz, 1H), 7.82 (d, J=2.2 Hz, 1H), 6.65 (dd, J=11.0, 17.5 Hz, 1H), 5.85 (d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com