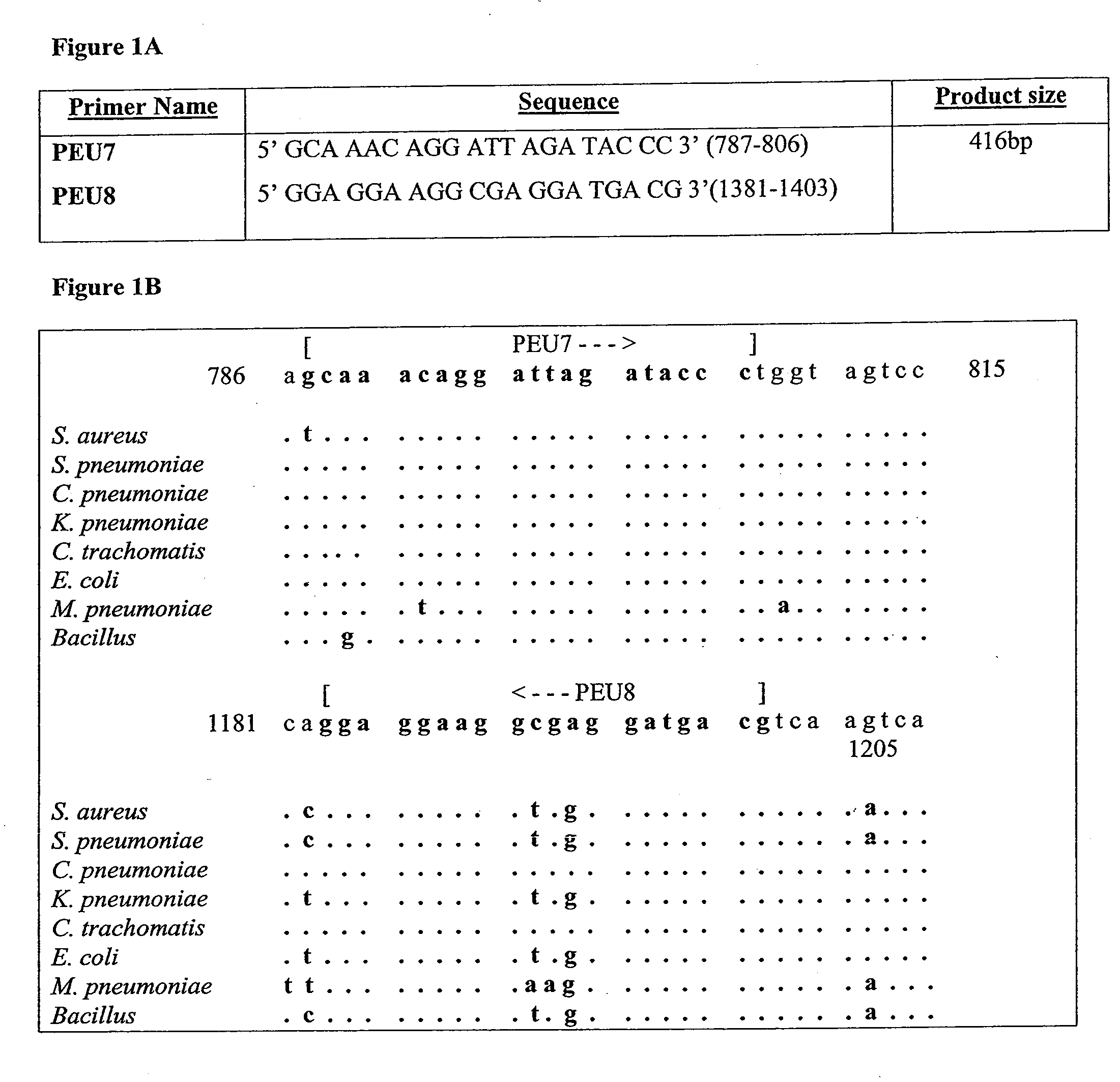
Molecular Diagnosis of Bacteremia
a bacteremia and molecular diagnostic technology, applied in the field of clinical diagnostics, can solve the problems of inability to quickly and accurately identify patients with bacterial infections, potential delays in diagnosis and treatment, and increased risk of morbidity and mortality, and achieve the effect of sensitive and accurate detection of bacterial microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study Design
[0029]This was a prospective identity-unlinked investigation. This technique, is a sampling method which allows single point in time patient related data collection with anonymous testing of patient blood samples taken for various blood borne pathogens. In brief, excess serum was retained from patients' 18 years of age and older who presented to The Johns Hopkins Hospital Emergency Department and had blood drawn for blood culture. Enrolled patients were assigned a unique study number, which was used to code the excess sera, as well as the laboratory and descriptive data. Descriptive data included demographics, clinical data, discharge diagnosis, and blood culture findings. After coding, all patient identifiers were stripped. In this way, results of the PCR analysis could not be directly linked to a patient by name or history number. The study was approved by the Johns Hopkins University Institutional Review Board.
Patients and Sample Collection
[0030]Practice at our hospit...
example 2
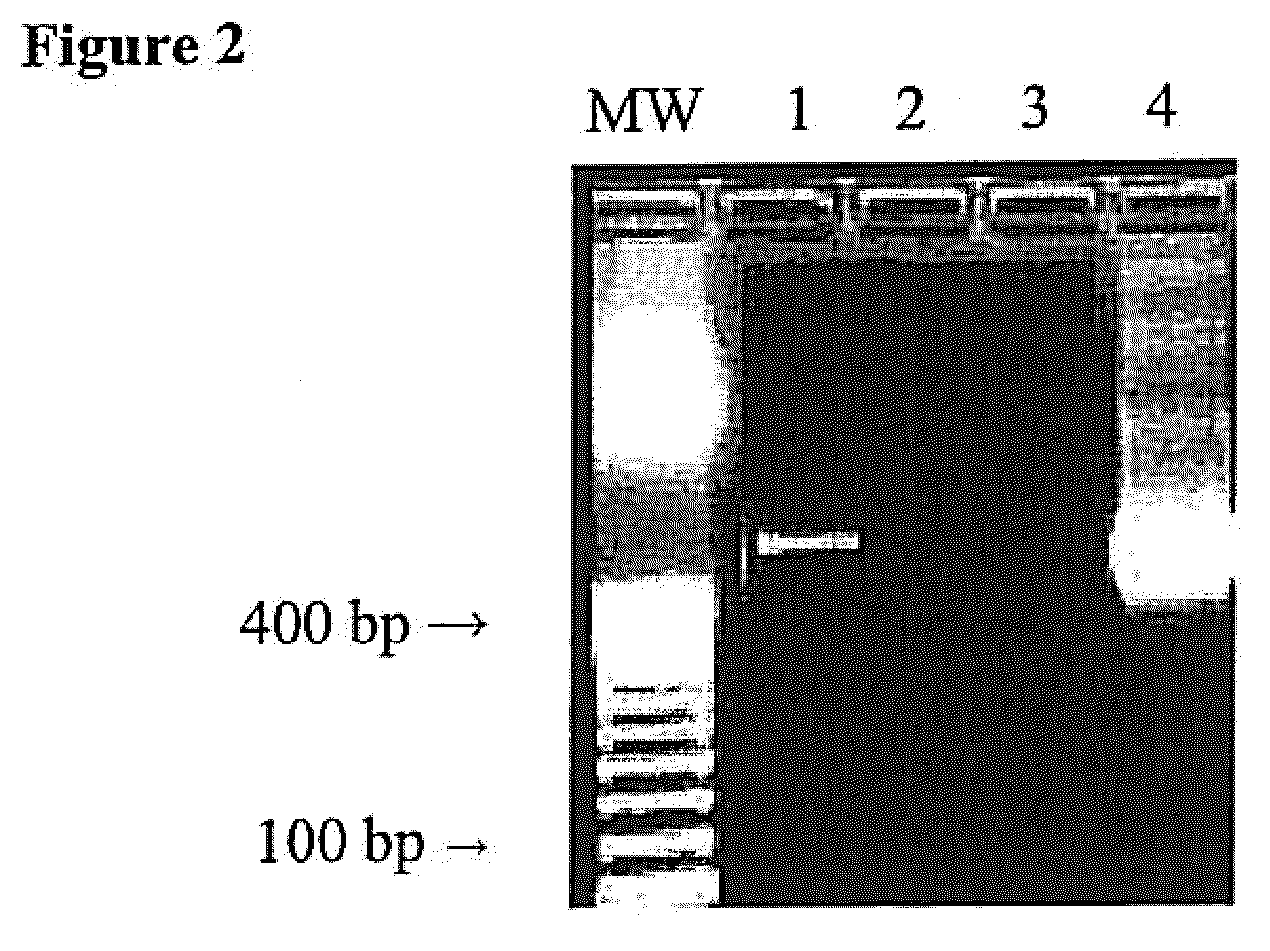
[0041]This example demonstrates the contamination present in PCR reagents and the efficacy of the subject method for destroying the contaminants.
[0042]The PCR amplification assay was first tested in a mock sample containing water, with no added bacteria to determine whether the reagents themselves contained contaminating sources of bacterial DNA that might lead to false-positive results. FIG. 2 shows results of a PCR amplification using the 16S rRNA primers described in FIG. 1. Lane 1 shows a product of the expected size, indicating that contaminant bacterial DNA is being amplified in our system. The effect of a predigestion step, in which AluI at is added to all components of the PCR cocktail is shown in Lane 2. (Titration of AluI from 1-20 U / reaction identified 10 U / reaction to be the optimal enzyme concentration.) Lane 3 shows results of a PCR amplification carried out using whole blood taken from a healthy control, with no bacteria added, again showing the absence of a signal. L...
example 3
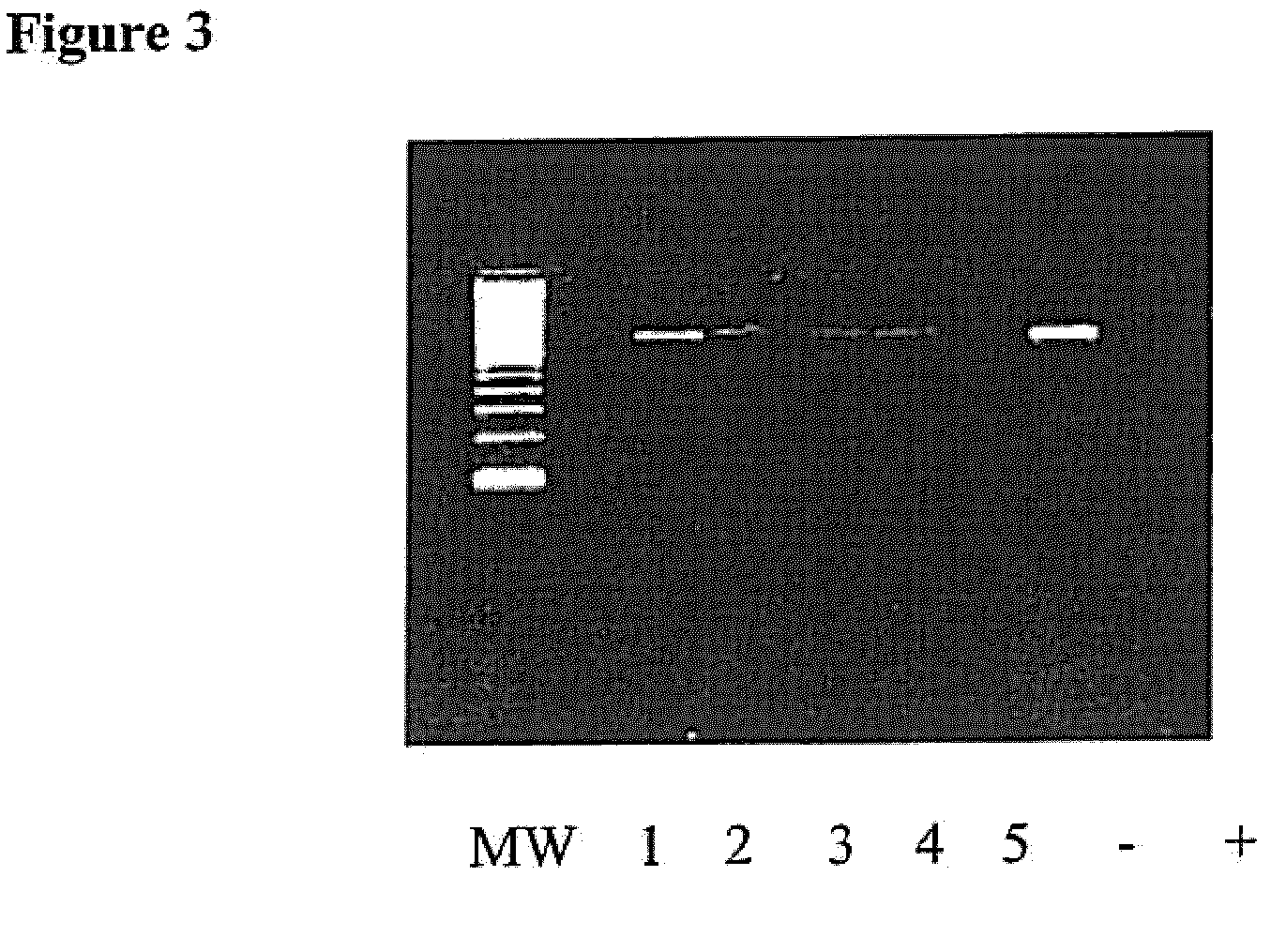
[0043]This example demonstrates the successful practice of the method using known bacteria spiked into test samples.
[0044]Healthy human whole blood was next spiked with 1 of 4 bacterial isolates and DNA was then extracted. A series of PCR amplifications reactions were subsequently carried out with the decontamination step, described above included, i.e., all PCR reagents were pretreated with the restriction enzyme, Alu I at 10 U / reaction prior to amplification. FIG. 3 shows the PCR amplified products from the whole blood specimens, as well as the negative control samples, in which no bacteria was added prior to PCR amplification. The amplified product of 411 base pairs was detected for each of the spiked reactions. DNA sequencing correctly confirmed the identity of each spiked pathogen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com