High penetration compositions and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a HPP from a Parent Drug
[0433]i) Preparation of a HPP from a Parent Drug which Contains at Least One Carboxylic Group.
[0434]In certain embodiments, the parent compound having the following Structure C:
[0435]is converted to a HPP having Structure A:
[0436]including stereoisomers and pharmaceutically acceptable salts thereof, wherein:
[0437]X is selected from the group consisting of nothing, O, P(O)OR1, NH, NR1 and S; and
[0438]each R1, R2, R3 and R4 are independently selected from the group consisting of nothing, H, CH2COOR6, substituted and unsubstituted alkyl, substituted and unsubstituted alkoxyl, substituted and unsubstituted perfluoroalkyl, substituted and unsubstituted alkyl halide, substituted and unsubstituted alkenyl, substituted and unsubstituted alkynyl, substituted and unsubstituted aryl, and substituted and unsubstituted heteroaryl groups, wherein any CH2 in R1, R2, R3 and R4 may be further independently replaced with O, S, P, NR1, or any other pharmaceutical...
example 2
The HPPs have Higher Aqueous Solubility Comparing to their Parent Drugs
[0515]HPPs have higher aqueous solubility comparing to their parent drugs (Table 1).
TABLE 1Solubility of NSAIAs and NSAIA-HPPsHPP(g / L)Parent Drug(g / L)diethylaminoethyl acetylsalicylate•AcOH in pH 7>300Aspirin0.01phosphate bufferdiethylaminoethyl 5-(2,4-difluorophenyl)salicylate•AcOH>400Diflunisal0.05in waterdiethylaminoethyl salicylsalicylate•AcOH>350Salsalate0.07diethylaminoethyl salicylate•AcOH>400salicylic acid0.1diethylaminoethyl 2-(ρ-isobutylphenyl) propionate•AcOH>300Ibuprofen0.05in waterdiethylaminoethyl 2-(3-benzoylphenyl)propionate•AcOH>450Ketoprofen0.1in waterdiethylaminoethyl 2-(3-phenoxyphenyl)propionate•AcOH>450Fenoprofen0.1diethylaminoethyl 2-(6-methoxy-2->450Naproxen0.1,naphthyl)propionate•AcOHdiethylaminoethyl α-methyl-4-(2->400Suprofen0.1thienylcarbonyl)benzeneacetate•AcOH,diethylaminoethyl α-methyl-(p-chlorobenzoyl)-5->450α-methyl-(p-0.2methoxy-2-methylindole 3-acetate•AcOH,chlorobenzoyl)-5-meth...
example 3
HPPs have Higher In Vitro Penetration Rates Across Human Skin Comparing to their Parent Drugs
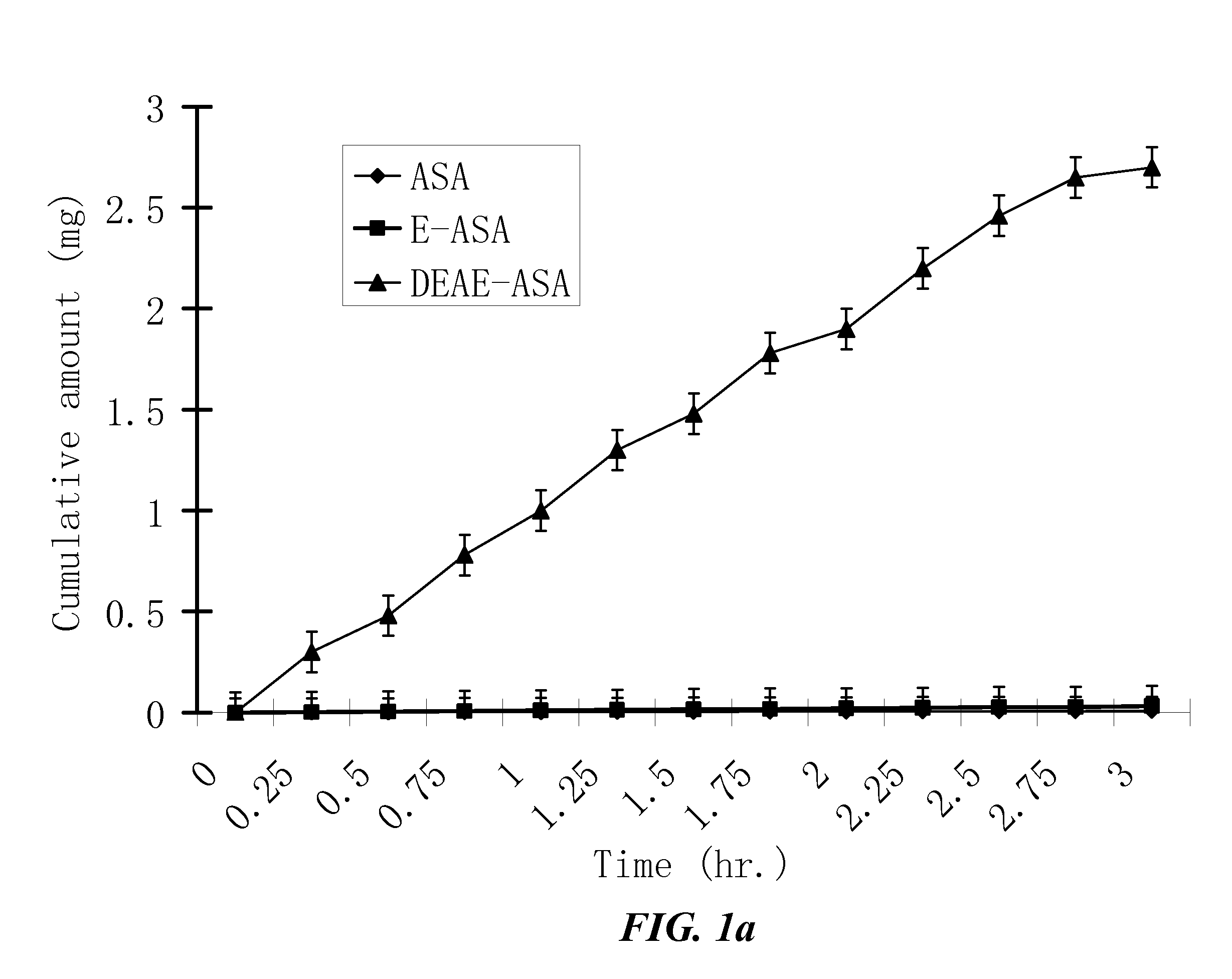
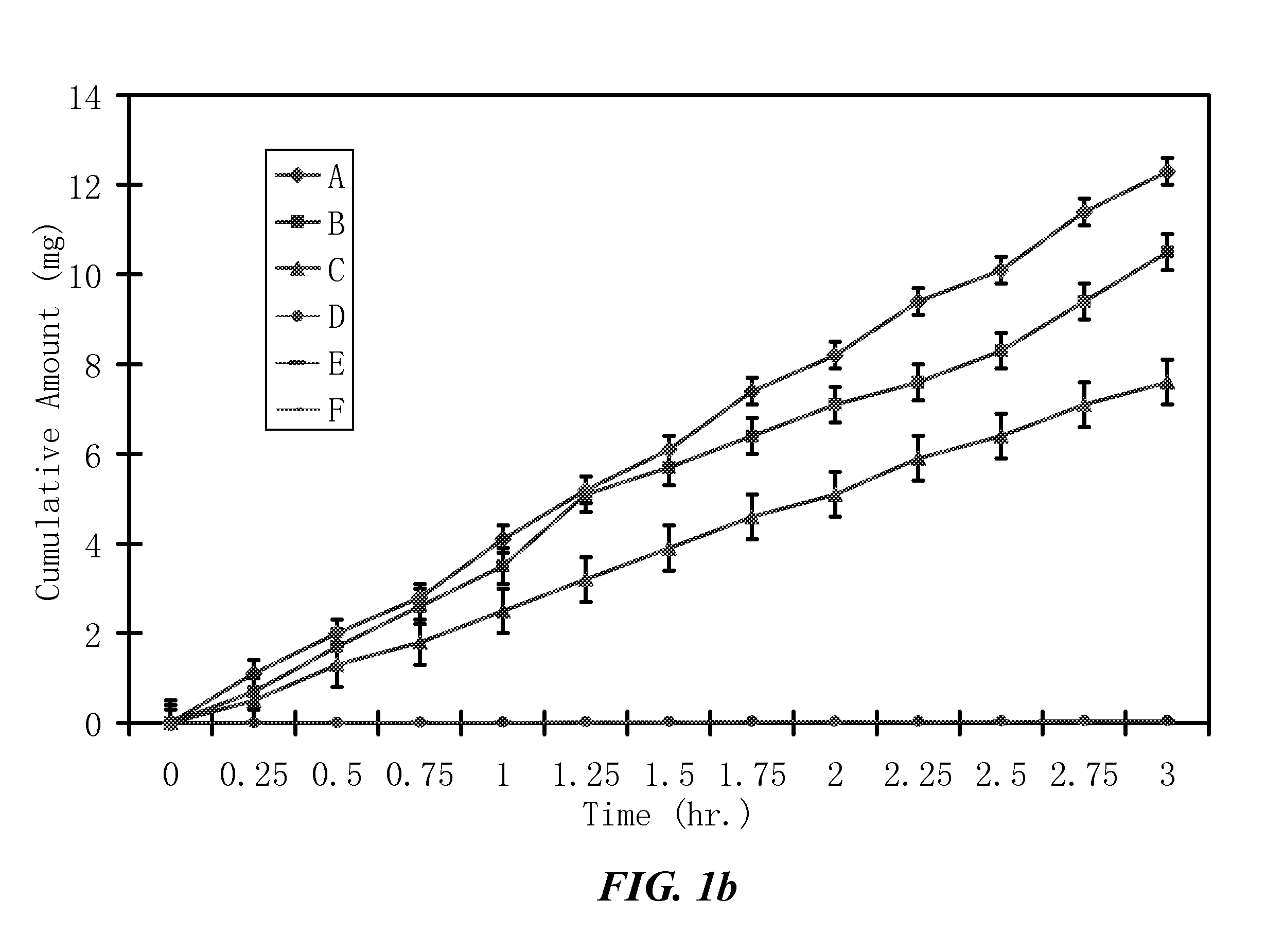
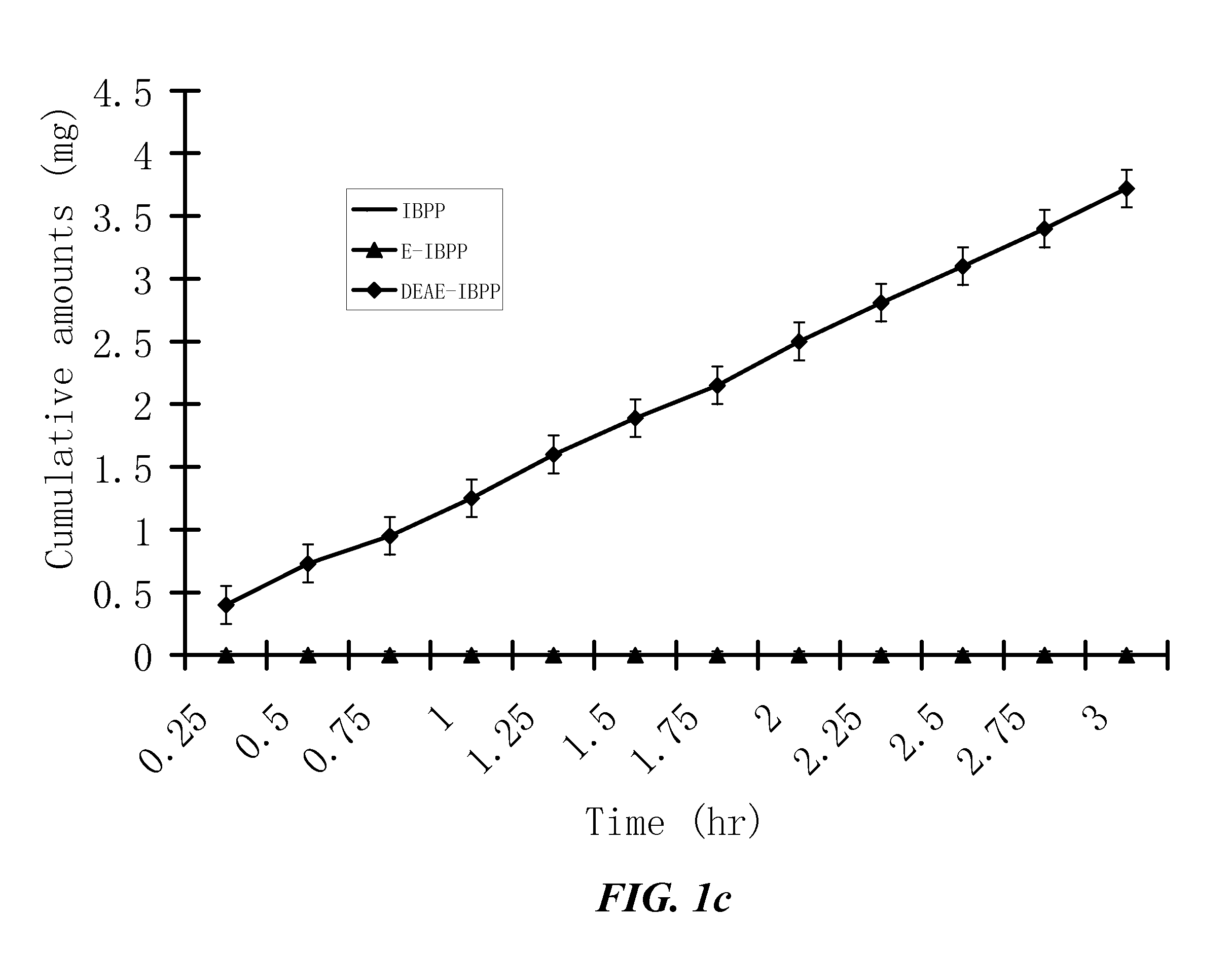
[0516]The penetration rates of HPPs and their parent drugs through human skin are measured in vitro by modified Franz cells. The Franz cells have two chambers, the top sample chamber and the bottom receptor chamber. The human skin tissue (360-400 μm thick) that separates the top and the receptor chambers is isolated from the anterior or posterior thigh areas.
[0517]The compound tested (2 mL, 20% in 0.2 M phosphate buffer, pH. 7.4) are added to the sample chamber of a Franz cell. The receptor chamber contains 10 ml of 2% bovine serum albumin in saline which is stirred at 600 rpm. The amount of the tested compound penetrating the skin is determined by high-performance liquid chromatography (HPLC) method. The results are shown in FIG. 1. The apparent flux values of the tested compounds are calculated from the slopes in FIG. 1 and summarized in Table 2.
[0518]Because the lowest detectable apparent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com