Topiramate Compositions and Methods of Making and Using the Same
a technology of sulfoalkyl ether and composition, which is applied in the field of compositions comprising topiramate and sulfoalkyl ether cyclodextrin, can solve the problems that the oral composition of topiramate may not be appropriate, and achieve the effect of blocking absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Topiramate Phase Solubility Study
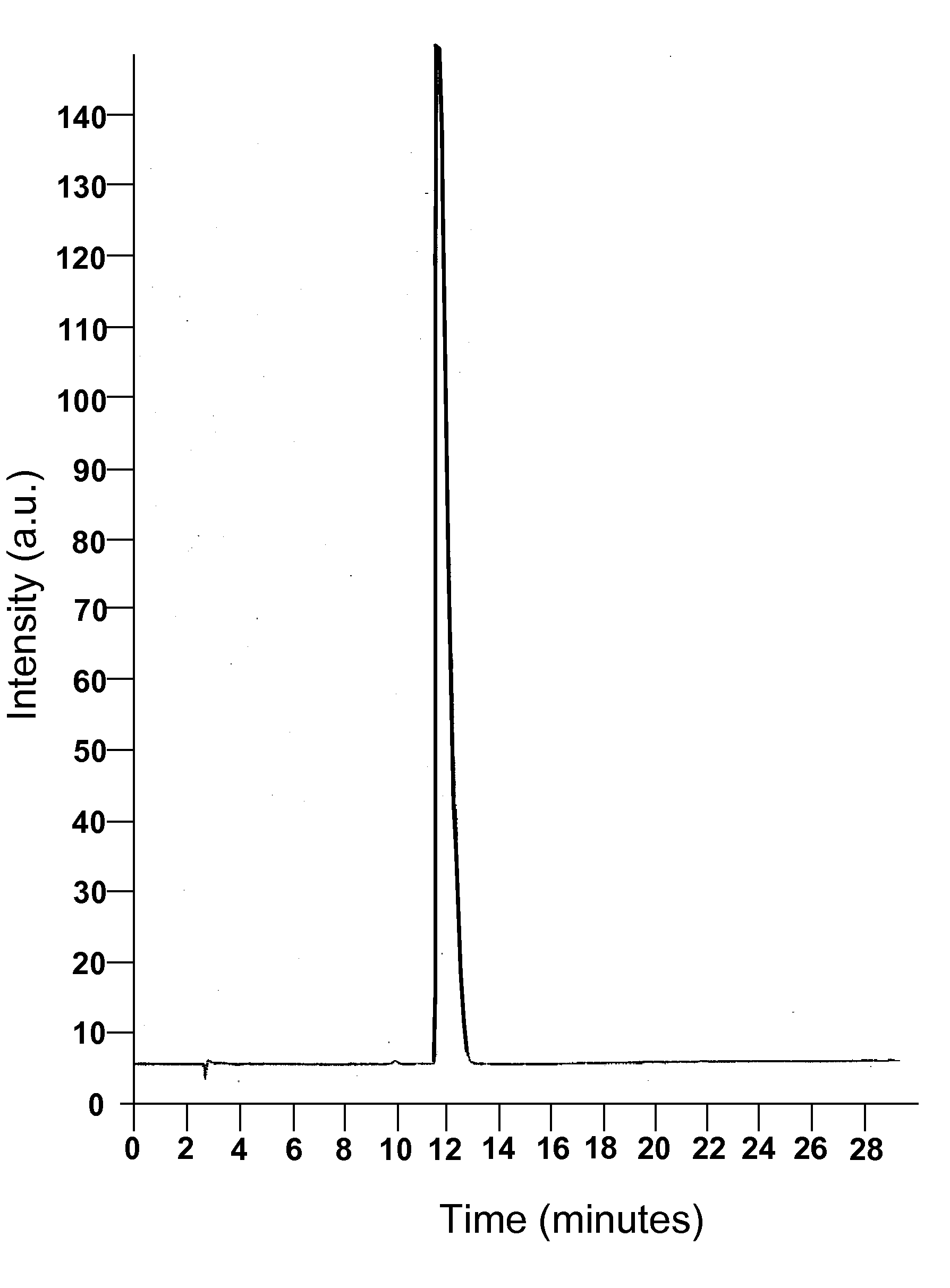
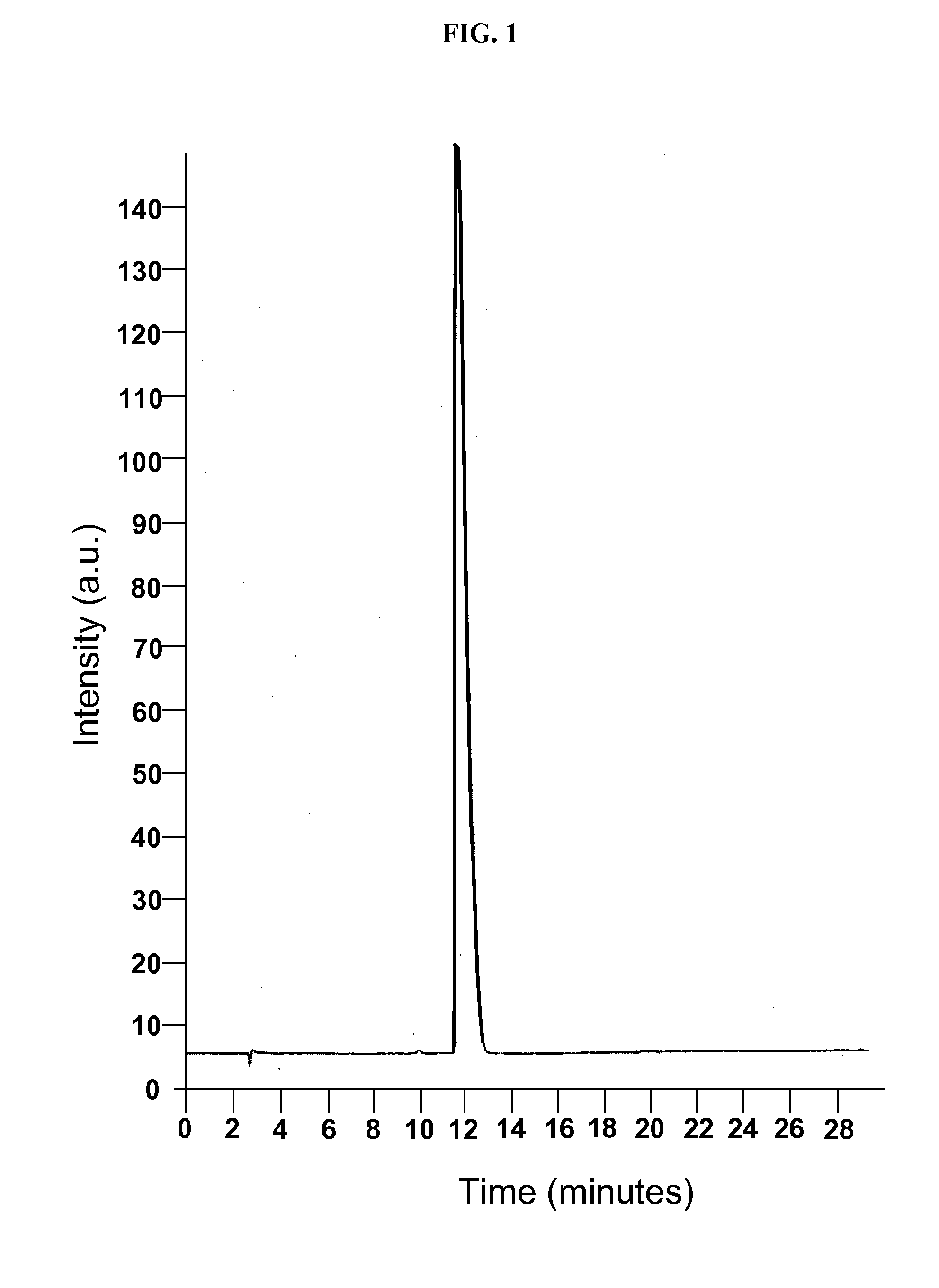
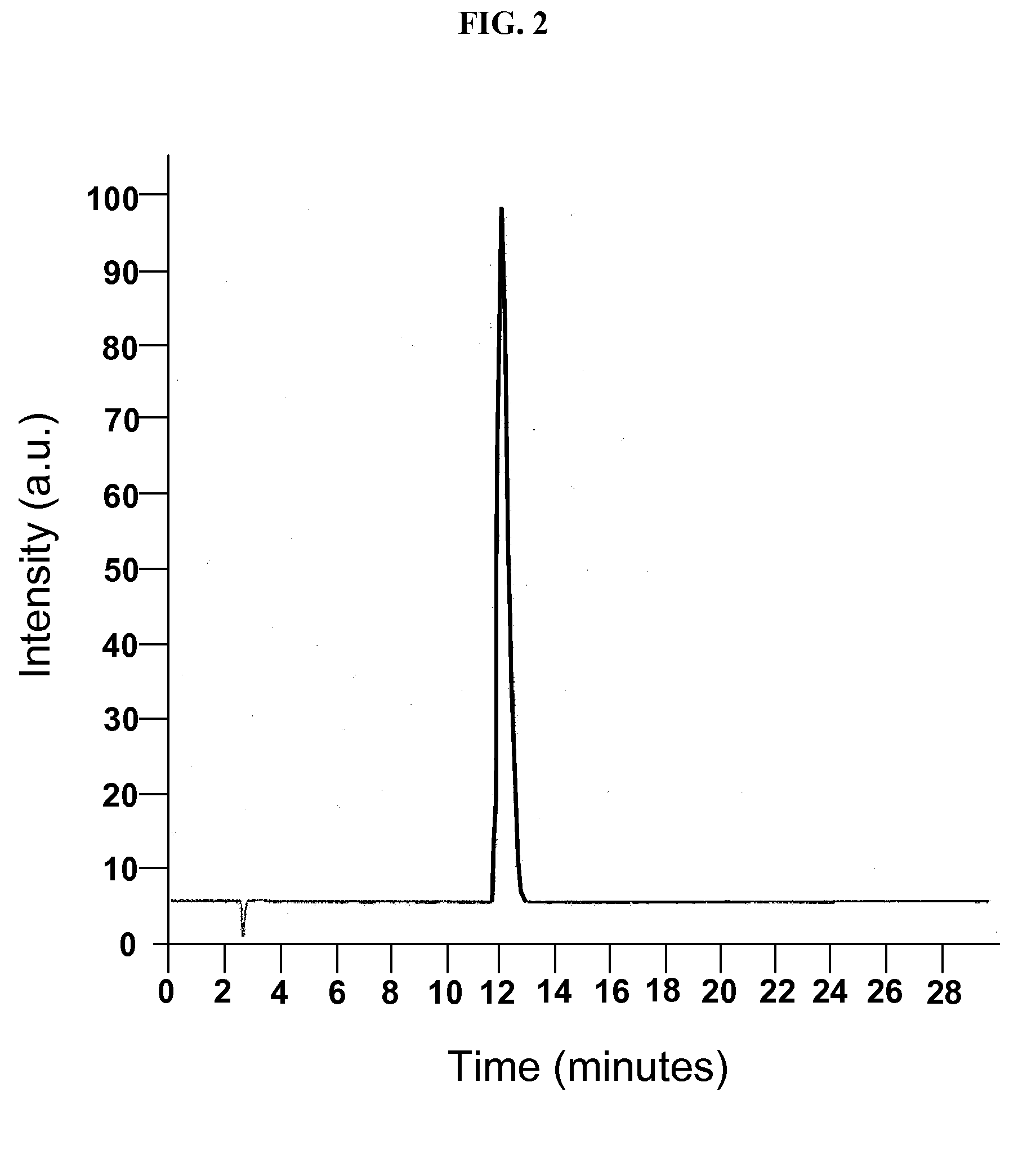
[0099]A phase solubility study was conducted with the cyclodextrin CAPTISOL® and topiramate to evaluate the extent of solubilization of the drug by the derivatized cyclodextrin. An HPLC method was modified from the literature and shown to be linear over the range of interest. Chromatograms from injection of two of the topiramate standards are shown in FIGS. 1 and 2.
[0100]Results of the solubility study are illustrated in FIGS. 3 and 4 and show that topiramate is well solubilized by the cyclodextrin CAPTISOL® in water. Type A-linear phase solubility is observed and a binding constant of 71 M−1 was calculated from the equation: K1:1=slope / S0(1-slope), where S0 is the intrinsic solubility of the drug and “slope” is the slope of the molar plot of drug solubility versus cyclodextrin content. The magnitude of the calculated binding constant is low due to the drug being reasonably soluble in water in the absence of cyclodextrin (intrinsic solubility of 7.86...
example 2
Preparation and Verification of [13C]6-Topiramate
[0110]Isotopically labeled topiramate ([13C]6-TPM) was synthesized by Isotech Laboratories, Inc. Quantitative identification of the stable-isotope topiramate was performed by Isotech Laboratories, Inc. using 1H-NMR, 13C-NMR, and mass spectrometry. The [13C]6-TPM was then to the University of Minnesota for further quantitative analysis by liquid chromatography / mass spectrometry (LC / MS).
[0111]The LC / MS procedure was as follows: 25 mg [13C]6-TPM was weighed on a Cahn electrobalance, transferred into a 2 dram vial, and dissolved in 2.5 mL of 10% w / v CAPTISOL® aqueous solution. The 10% w / v CAPTISOL® aqueous solution was prepared by weighing 10 g of CAPTISOL® (adjusted for water content) and dissolving it in 100 mL of water. Reference unlabeled topiramate (obtained from Sigma-Aldrich Co. or Toronto Research Chemicals, Inc.) was prepared in an identical fashion. Separation of topiramate was performed using reverse phase chromatography, and d...
example 3
Formulation of [13C]6-TPM with Sulfoalkyl Ether Cyclodextrin
[0113]The stable-isotope topiramate was sent in sealed containers from University of Minnesota to the Pharmaceutical Service Division, College of Pharmacy, University of Iowa, Iowa City, 10 52242, for formulation into a parenteral solution suitable for administration into humans.
[0114]The stable-isotope topiramate was formulated with CAPTISOL® for intravenous administration. The resulting composition contained 1% w / v topiramate and 10% w / v CAPTISOL®. The manufacturing procedure was as follows:[0115]1) 200 g of CAPTISOL® (adjusted for water content) was dissolved in 2.0 L of deionized sterile water to generate a 10% w / v CAPTISOL® solution;[0116]2) 20 g of [13C]6-TPM was added to the 10% w / v CAPTISOL® solution;[0117]3) The solution was stirred for 24 hours at room temperature;[0118]4) Ampoules were sterilized in preparation for filling;[0119]5) All equipment to be used was prepared and sterilized;[0120]6) The solution was tra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com