Method for electrostatic coating of a medical device
a medical device and electrostatic coating technology, applied in the field of electrostatic coating a medical device, can solve the problems of wasting a large amount of coating material, affecting the safety of operators and the environment, and hazardous for the operator and the environment, and achieve the effect of reducing the risk of contamination, and reducing the safety of users
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
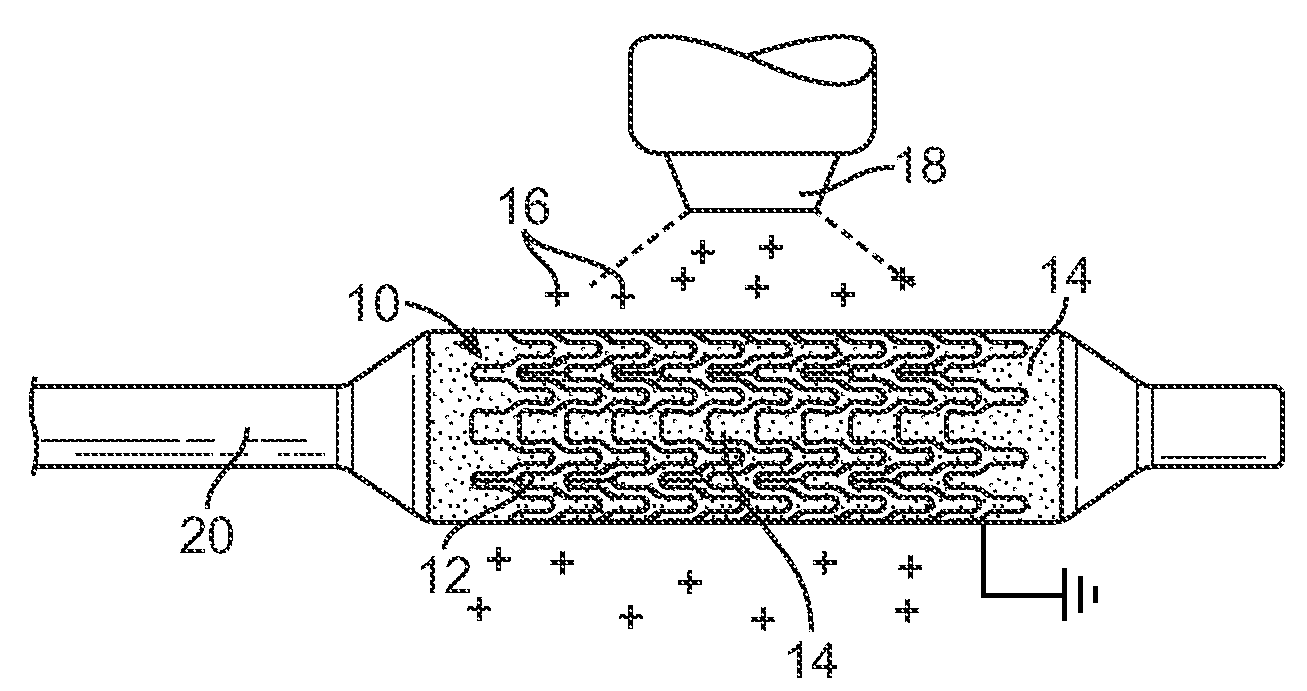
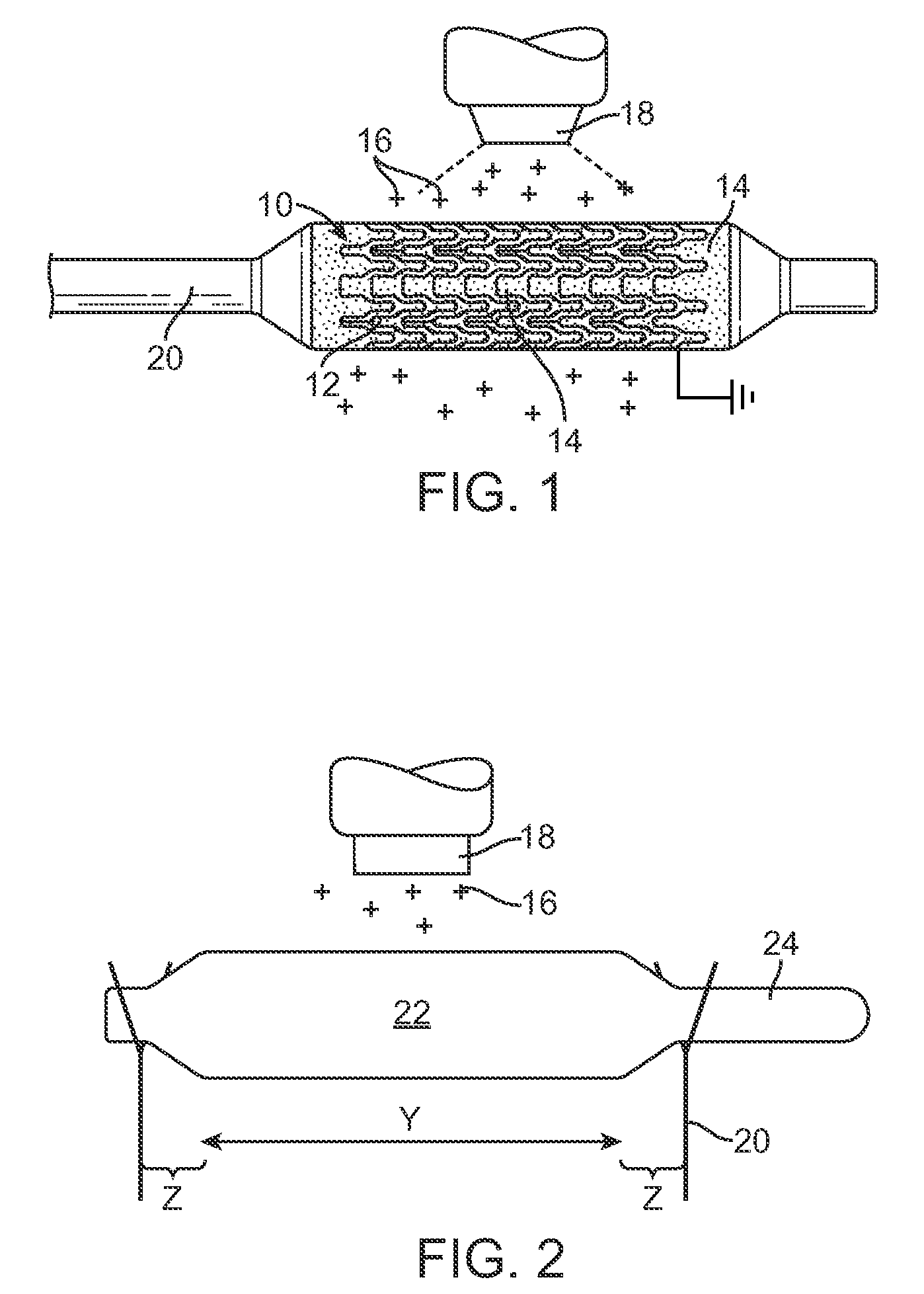
[0022]A method is provided for electrostatically coating stents having a polymeric component. As illustrated in FIG. 1, a stent 10 is supported by a mandrel or support structure 20. The mandrel or support structure 20 can be coupled to a driving means to provide rotational motion for spinning the stent 10 during electrostatic deposition. In one embodiment, the stent 10 can have a hollow, tubular body, including struts separated by gaps, as best illustrated by reference number 12 and 14, respectively. In other embodiments, the stent can be made from wires, fibers, coiled sheet, with or without gaps, or a scaffolding network of rings connected by arms. The stent can have any particular geometrical configuration, such as sinusoidal strut configuration, and should not be limited to what is illustrated in FIG. 1. The stent can be balloon expandable or self-expandable, both of which are well known in the art. The stent is preferably for cardiovascular use. In some embodiments, the stent c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com