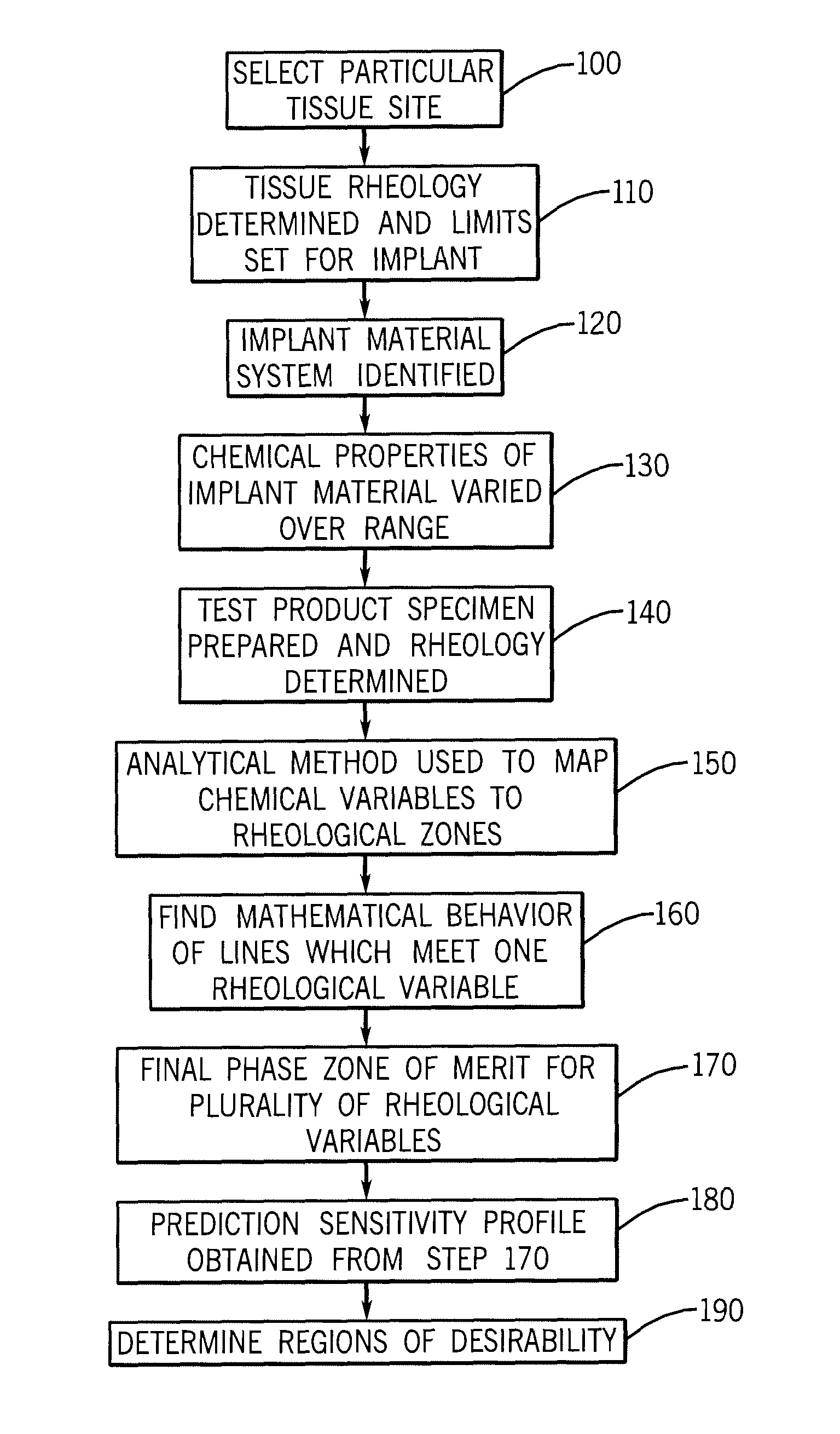
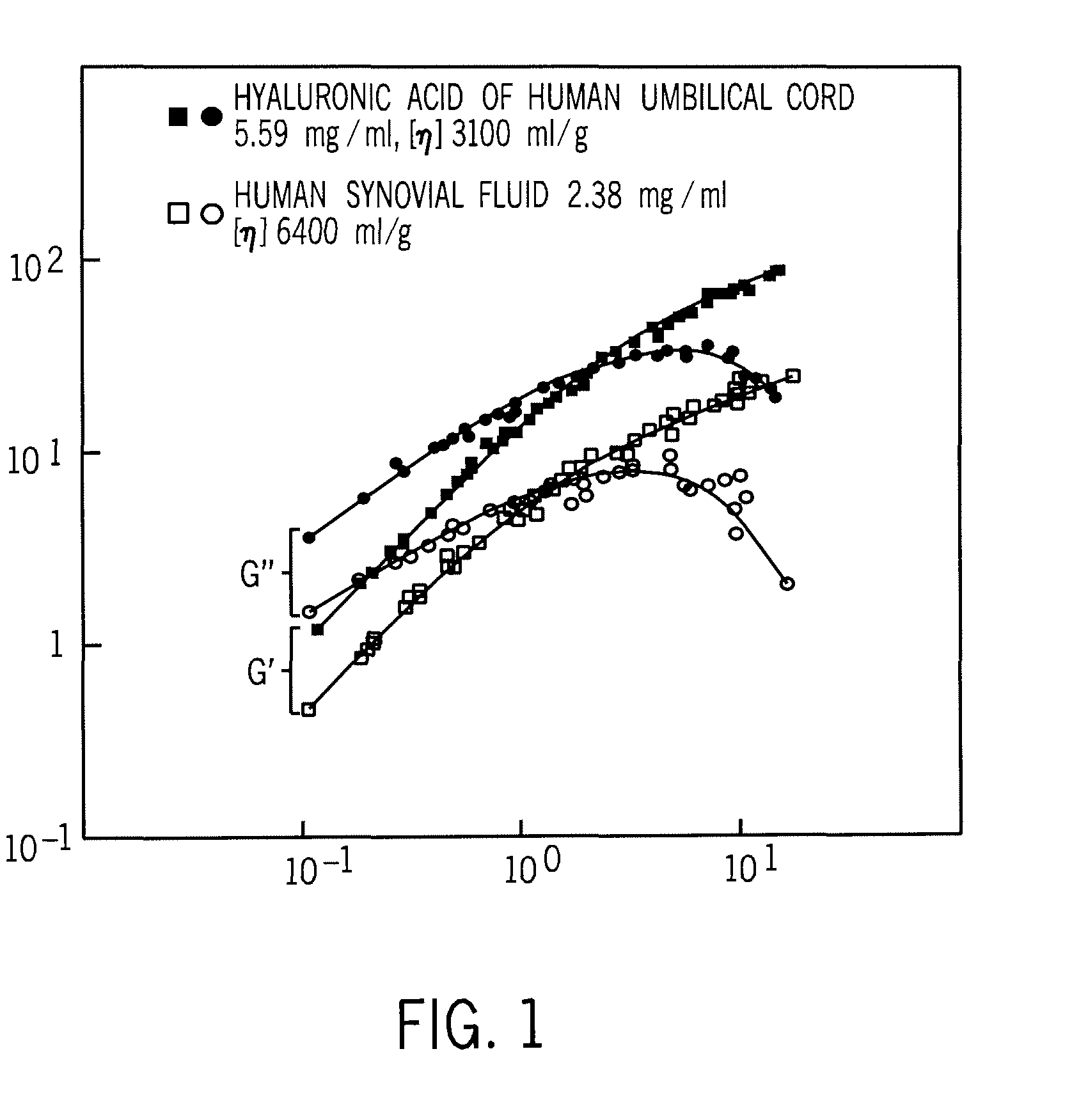
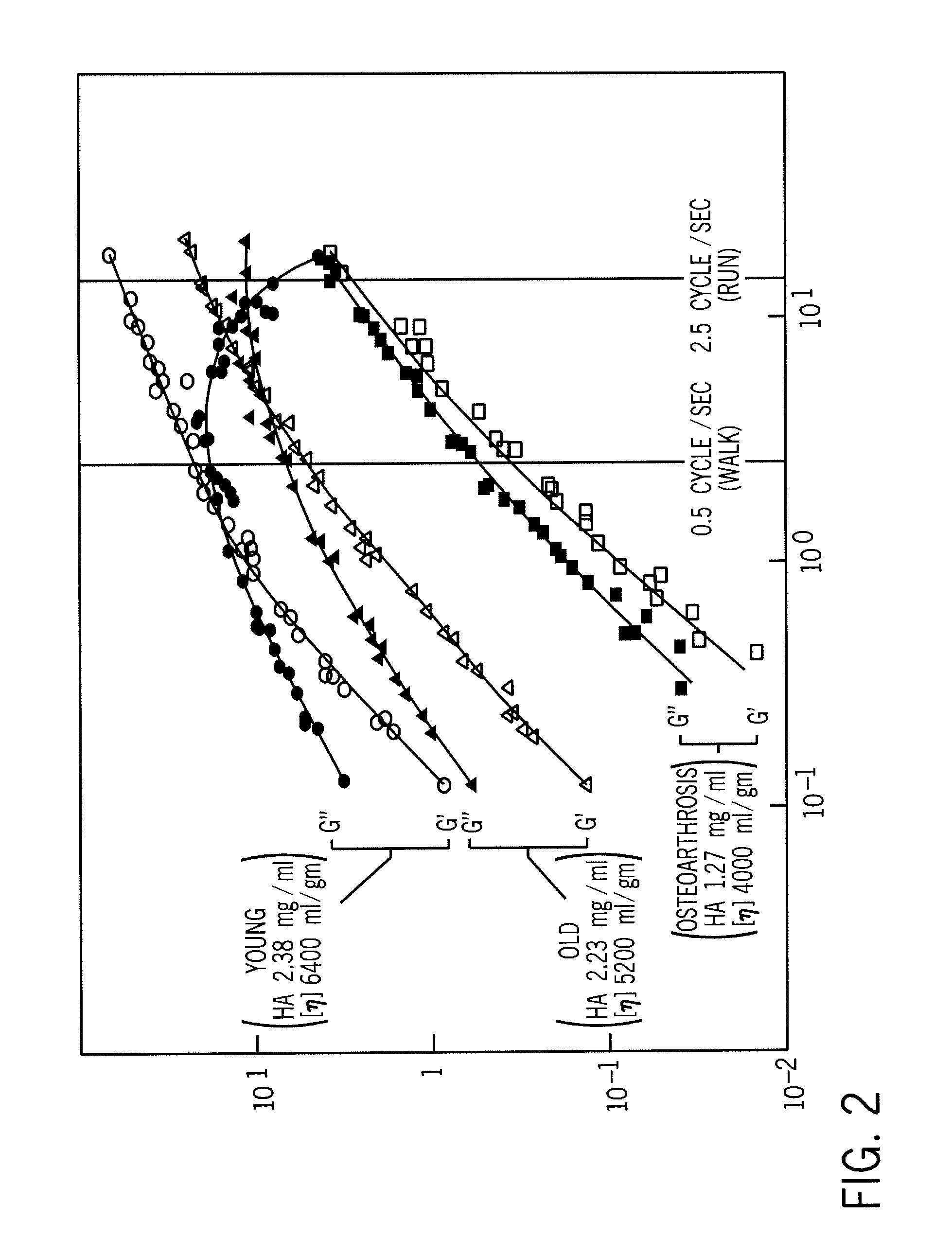
Implantation Compositions for Use in Tissue Augmentation
a composition and tissue technology, applied in the field of tissue augmentation, can solve the problems of withdrawn from commercial application, damaged bovine collagen dispersion, and failure of conventional methods and products to address several problems with current gels, and achieve the effects of specific compatibility and stability, minimizing immuno-histo tissue response, and improving mechanical and visual appearan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2.3% Sodium CMC Gel in Sterile Water.
[0107]Sodium carboxymethylcellulose was prepared in sterile water for injection and adjusted to a pH of from about 7.1 to about 8.0 using potassium hydroxide. The dispersion was mixed in an orbital Ross mixer@1725 RPM for 5 minutes followed by mixing in an orbital Ross mixer@1725 RPM for 40 minutes. while holding a vacuum@26 mm Hg or more. The composition was then steam sterilization at 121° C. for times ranging from 3 minutes to 30 minutes. In addition, one sample was sterilized for time intervals between 3 minutes and 30 minutes@121° C. Results are shown in FIG. 10 where G′ represents the elastic modulus, G″ represents the viscous modulus and η the complex viscosity. The profile shows that G′ and G″ intersect at 0.495 Hz (3.2 Rad / sec). Above this frequency, the composition exhibits non-Newtonian solution characteristics (tan δ<1.0).
example 2
Preparation of 2.4% Sodium CMC Gel in Sterile Water.
[0108]Sodium carboxymethylcellulose was prepared in sterile water for injection and adjusted to a pH of from about 7.1 to about 8.0 using potassium hydroxide. The dispersion was mixed in an orbital Ross mixer@1725 RPM for 5 minutes followed by mixing in an orbital Ross mixer@1725 RPM for 40 minutes while holding a vacuum@26 mm Hg or more. The composition was then steam sterilization at 121° C. for times ranging from 3 minutes to 30 minutes. In addition, one sample was sterilized for time intervals between 3 minutes and 30 minutes@121° C. Results are shown in FIG. 11 where G′ represents the elastic modulus, G″ represents the viscous modulus and μ the complex viscosity. The profile shows that G′ and G″ intersect at 0.0299 Hz (1.8 Rad / sec) (lower frequency than that shown in FIG. 1). Above this frequency, the composition exhibits non-Newtonian solution characteristics (tan δ<1.0).
example 3
Preparation of 2.5% Sodium CMC Gel in Sterile Water.
[0109]Sodium carboxymethylcellulose was prepared in sterile water for injection and adjusted to a pH of from about 7.1 to about 8.0 using potassium hydroxide. The dispersion was mixed in an orbital Ross mixer@1725 RPM for 5 minutes followed by mixing in an orbital Ross mixer@1725 RPM for 40 minutes. while holding a vacuum@26 mm Hg or more. The composition was then steam sterilization at 121° C. for times ranging from 12 minutes to 30 minutes. In addition, one sample was sterilized for time intervals between 3 minutes and 12 minutes@121° C. Results are shown in FIG. 12 where G′ represents the elastic modulus, G″ represents the viscous modulus and η the complex viscosity. The profile shows that G′ and G″ intersect at 0.157 Hz (1 rad / sec) frequency than shown in FIGS. 10 and 11. Above this frequency, the composition exhibits non-Newtonian solution characteristics (tan δ<1.0).
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| frequency response | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com