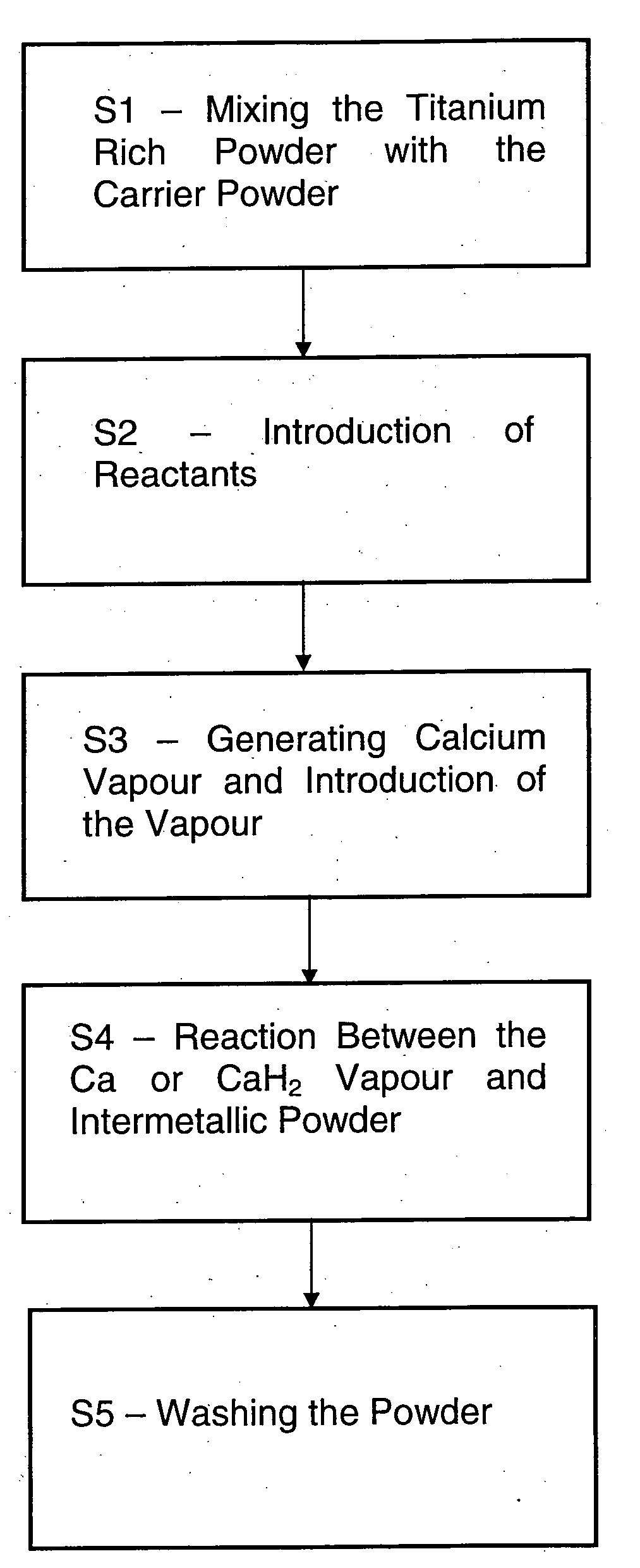
Method for purification of metal based alloy and intermetallic powders
a technology of metal which is applied in the field of purification of titanium based can solve the problems of limiting the range of practical applications of metals, deteriorating mechanical and other properties of articles to an unacceptable level, and not being applied to active metals (such as titanium) based metals, alloys and intermetallic powders. , to achieve the effect of low cost, high purity and low grade calcium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
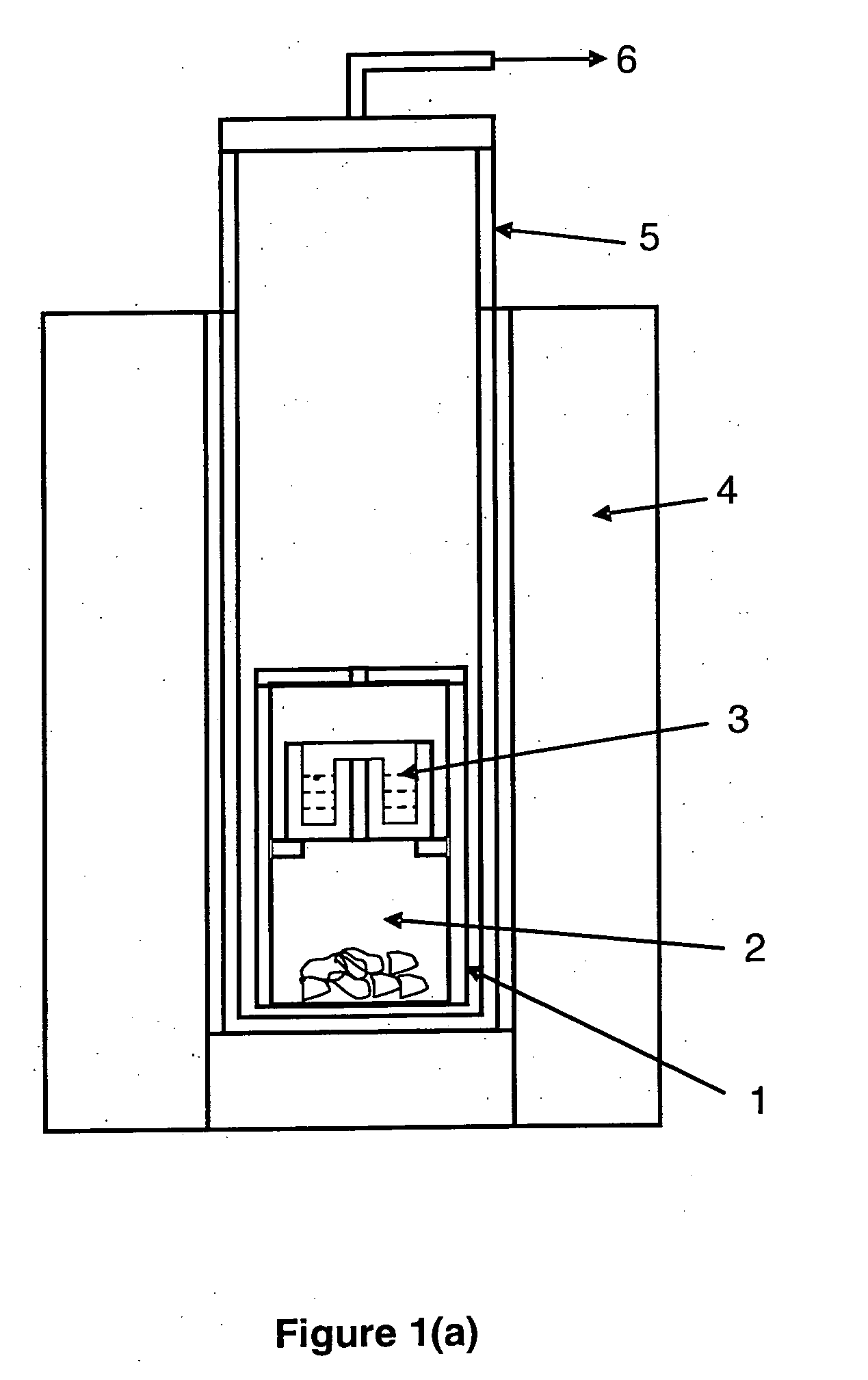
[0081]1 gram of TiAl(O) powder with a composition of approximately 69.6 wt % Ti-26.7 wt % Al-3.7 wt % O and containing Al2O3 inclusions was homogeneously mixed with 1.8 grams of CaCl2.2H2O powder. The X-ray diffractometry (XRD) pattern as shown in FIG. 2 shows that the powder mainly consists of TiAl(O), Ti3Al(O) and Al2O3 phases. Here, O in the brackets represents the dissolved oxygen. FIG. 3 show the scanning electron microscopy (SEM) images of the cross sections of the powder particles. As shown in FIG. 3, Al2O3 inclusions are embedded in the TiAl based powder particles. The mixture of the TiAl(O) powder and the CaCl2.2H2O powder was placed in the top chamber of the reaction container as shown schematically in FIG. 1(a). 1 gram of calcium granules with a purity of 99% were placed in the bottom chamber of the reaction container. The container was covered with a lid which has a small opening. The reaction container was placed in a stainless steel retort.
[0082]The retort was then sea...
example 2
[0083]1 gram of Ti—Al—V alloy powder with a composition of approximately 80 wt % Ti-10 wt % Al-5 wt % V-5 wt % O and containing Al2O3 inclusions was homogeneously mixed with 1.8 grams of CaCl2.2H2O powder. The XRD pattern as shown in FIG. 6 shows that the powder mainly consists of Ti(Al,V,O), Ti3Al(O) and Al2O3 phases. Here, Al, V and O in the brackets represent Al, V and O dissolved in the titanium rich metallic and intermetallic phased. FIG. 7 shows the SEM images of the cross sections of the powder particles. As shown in FIG. 7, Al2O3 inclusions are embedded in the Ti—Al—V based powder particles. The mixture of the Ti—Al—V powder and the CaCl2.2H2O powder was placed in the top chamber of the reaction container as shown schematically in FIG. 1(a). 1 gram of calcium granules with a purity of 99% were placed in the bottom chamber of the reaction container. The container was covered with a lid which has a small opening. The reaction container was placed in a stainless steel retort.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com