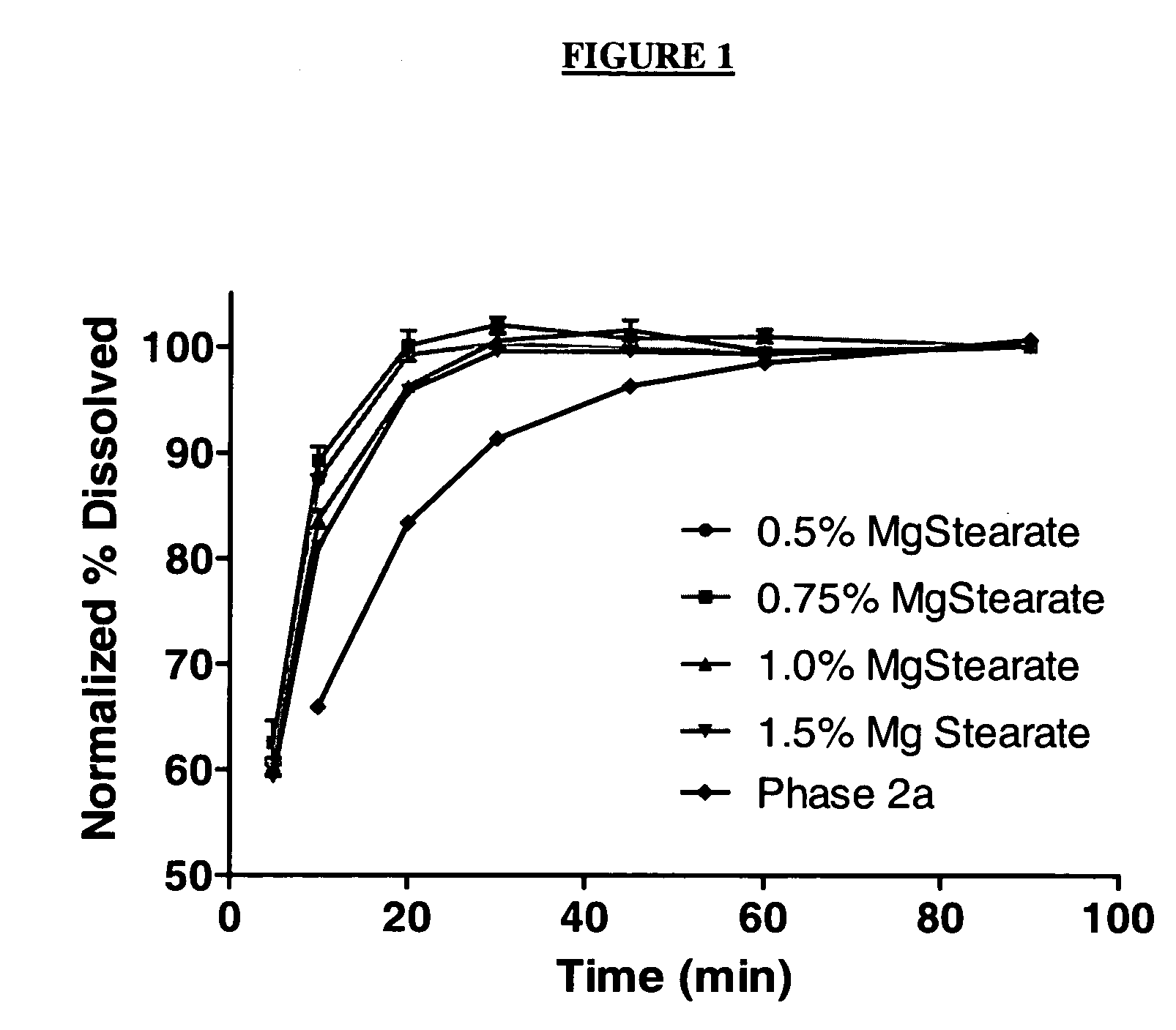
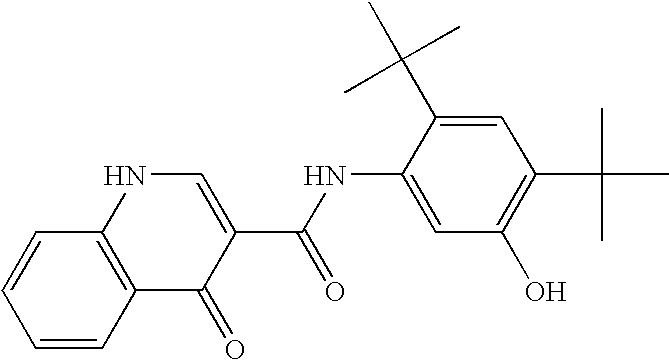
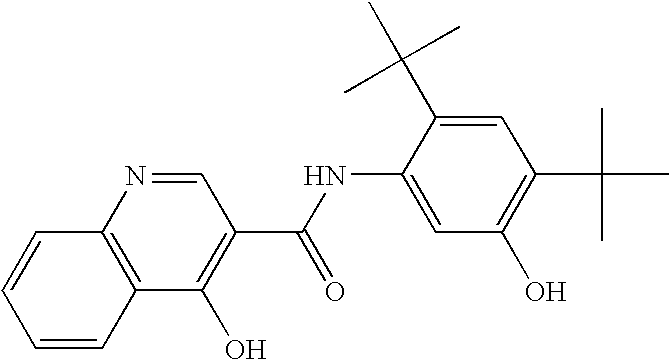
Pharmaceutical composition and administration thereof
a technology of pharmaceutical compositions and compositions, applied in the field of pharmaceutical compositions, can solve the problems of imbalance in ion and fluid transport, no cure, and individuals with two copies of the cf associated gene suffering from the debilitating and fatal effects of cf, and achieve the effects of enhancing its visual appeal, taste, and scen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Exemplary Tablet 1 (Formulated to have 25 mg of Compound 1)
[0699]A batch of round core ⅜″ tablets was formulated to have approximately 25 mg of Compound 1 per tablet using the amounts of ingredients recited in Table 1, below.
TABLE 1Ingredients for Exemplary Tablet 1.Percent DoseDoseBatchTablet Formulation% Wt. / Wt.(mg)(g)Intermediate A15.29%51.23512.5Microcrystalline cellulose35.00%117.251172Lactose43.85%146.001460Sodium croscarmellose5.000%16.75167.5SLS0.500%1.67516.75Colloidal silicon dioxide0.125%0.41884.188Magnesium stearate0.50%1.67516.75Total100%3353350
[0700]Intermediate A, microcrystalline cellulose (FMC MCC Avicel® PH102, commercially available from FMC BioPolymer Corporation of Philadelphia, Pa.), lactose (Foremost FastFlo® Lactose #316 commercially available from Foremost Farms USA of Baraboo, Wis.), sodium croscarmellose (FMC Ac-Di-Sol®, commercially available from FMC BioPolymer Corporation of Philadelphia, Pa.), SLS, and colloidal silicon dioxide (Cabot Cab-O-Sil® M-5P F...
example 2
Exemplary Tablet 2 (Formulated to have 50 mg of Compound 1)
[0710]A batch of round core ⅜″ tablets was formulated to have about 50 mg of Compound 1 per tablet using the amounts of ingredients recited in Table 2, below.
TABLE 2Ingredients for Exemplary Tablet 2.Percent DoseDoseBatchTablet Formulation% Wt. / Wt.(mg)(g)Intermediate A30.60%102.501025.0Microcrystalline cellulose25.00%83.75837.5Lactose38.28%128.231282.3Sodium croscarmellose5.000%16.75167.5SLS0.500%1.67516.75Colloidal silicon dioxide0.125%0.41884.188Magnesium stearate0.50%1.67516.75Total100%3353350
[0711]Intermediate A, microcrystalline cellulose, lactose, sodium croscarmellose, SLS, and colloidal silicon dioxide were sieved through a 20 mesh screen to remove lumps, and each of the sieved ingredients was added to a 16 quart V-blender in the following order:
[0712]1) lactose;
[0713]2) SLS;
[0714]3) sodium croscarmellose;
[0715]4) colloidal silicon dioxide;
[0716]5) Intermediate A; and
[0717]6) microcrystalline cellulose PH101
[0718]The...
example 3
Exemplary Tablet 3 (Formulated with PVP / VA Polymer to have 150 mg of Compound 1)
[0720]A batch of caplet-shaped tablets was formulated to have about 150 mg of Compound 1 per tablet using the amounts of ingredients recited in Table 3, below.
TABLE 3Ingredients for Exemplary Tablet 3.Percent DoseDoseBatchTablet Formulation% Wt. / Wt.(mg)(g)Intermediate B40.000%187.50240.00Microcrystalline cellulose27.063%126.86162.38Lactose27.063%126.86162.38Sodium croscarmellose3.000%14.0618.00SLS0.500%2.343.00Colloidal silicon dioxide1.000%4.696.00Coloring0.375%1.762.25Magnesium stearate1.000%4.696.00Total100%469600
[0721]A glidant blend of colloidal silicon dioxide (Cabot Cab-O-Sil® M-5P Fumed Silicon Dioxide) and SLS was produced by hand mixing these two ingredients, in the amounts given in Table 3, and filtering the resulting mix through a 70 mesh screen sieve. A color blend of coloring (Colorcon Blue #1 Aluminum Lake #5516) and sodium croscarmellose (FMC Ac-Di-Sol®) was produced by hand mixing these ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com