Antibacterial nanofiber
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0132]Ten parts by weight of polylactic acid resin (LACEA H280, available from Mitsui Chemicals, Inc.) and 45 parts by weight of dimethylformamide (abbreviated below as “DMF”) were mixed and heated to 60° C., thereby dissolving the polylactic acid resin in the DMF and obtaining 55 parts by weight of a polylactic acid-containing solution (solids content, 18 wt %).
[0133]This lactic acid-containing solution (spinning dope) was placed in a syringe and electrostatic spinning was carried out at a discharge tip orifice diameter of 0.4 mm, an applied voltage of 20 KV (at room temperature and atmospheric pressure), and a distance from the discharge tip orifice to the fibrous substance collecting electrode of 15 cm, thereby giving a nanofiber nonwoven fabric.
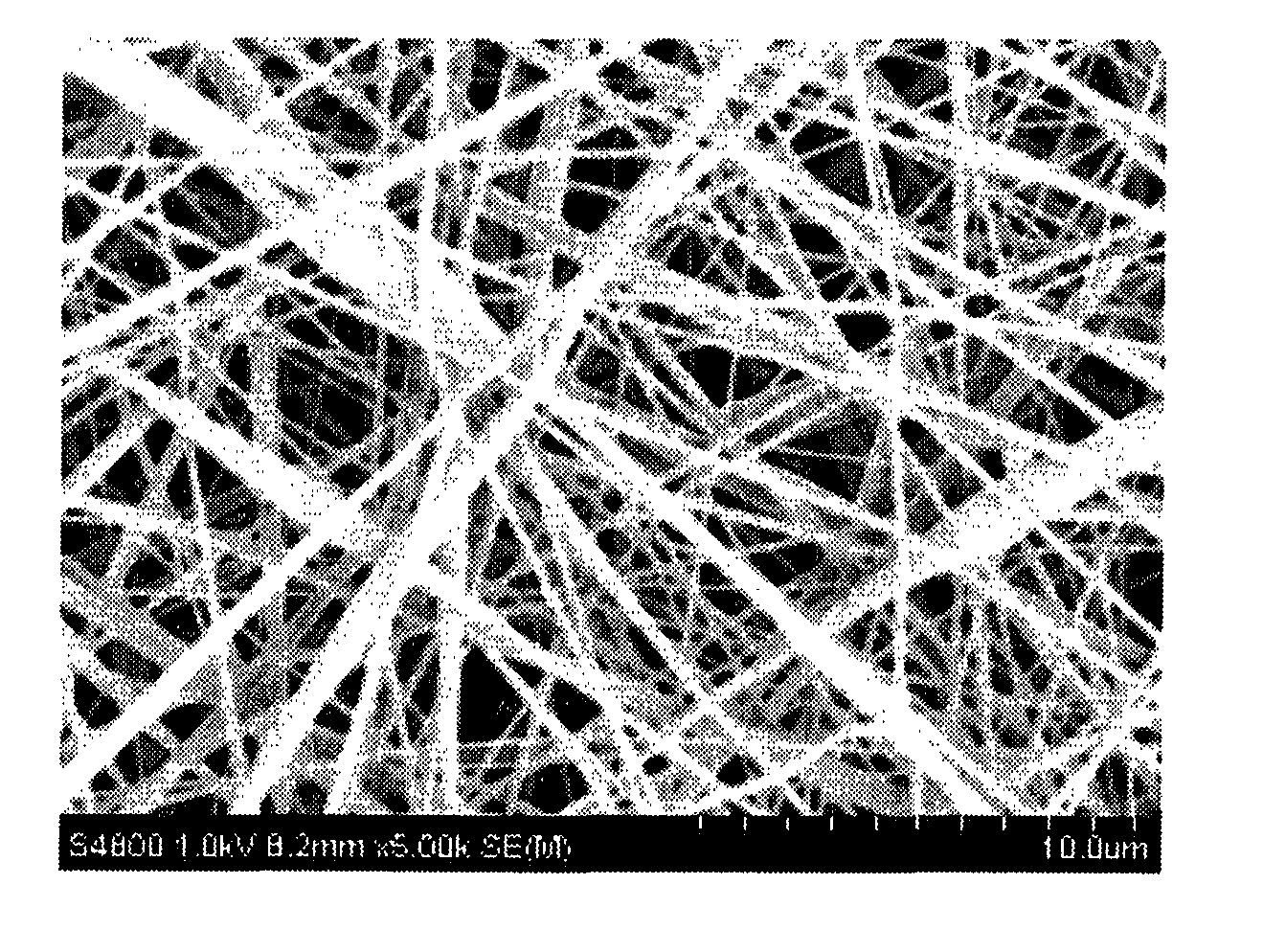
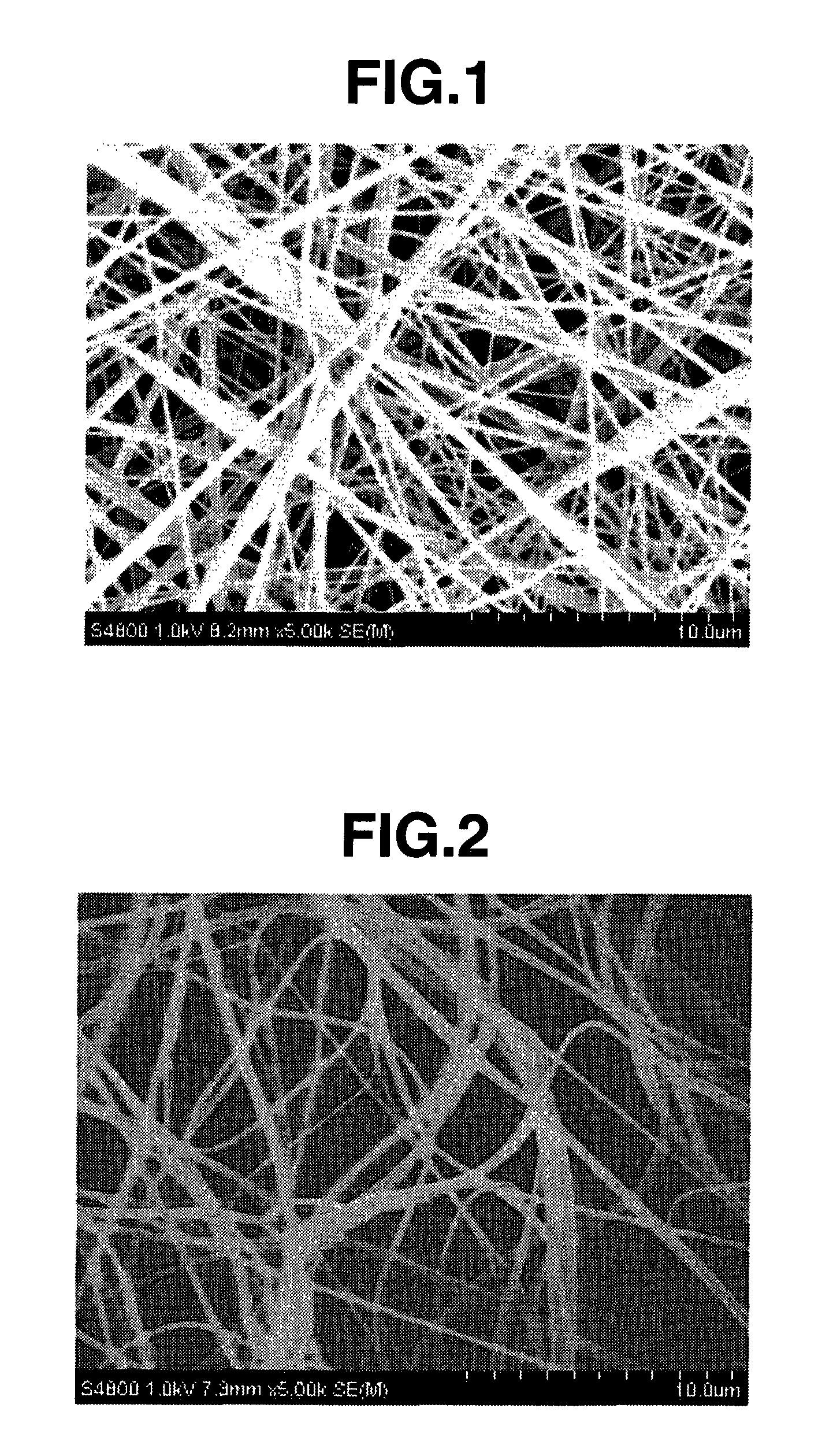
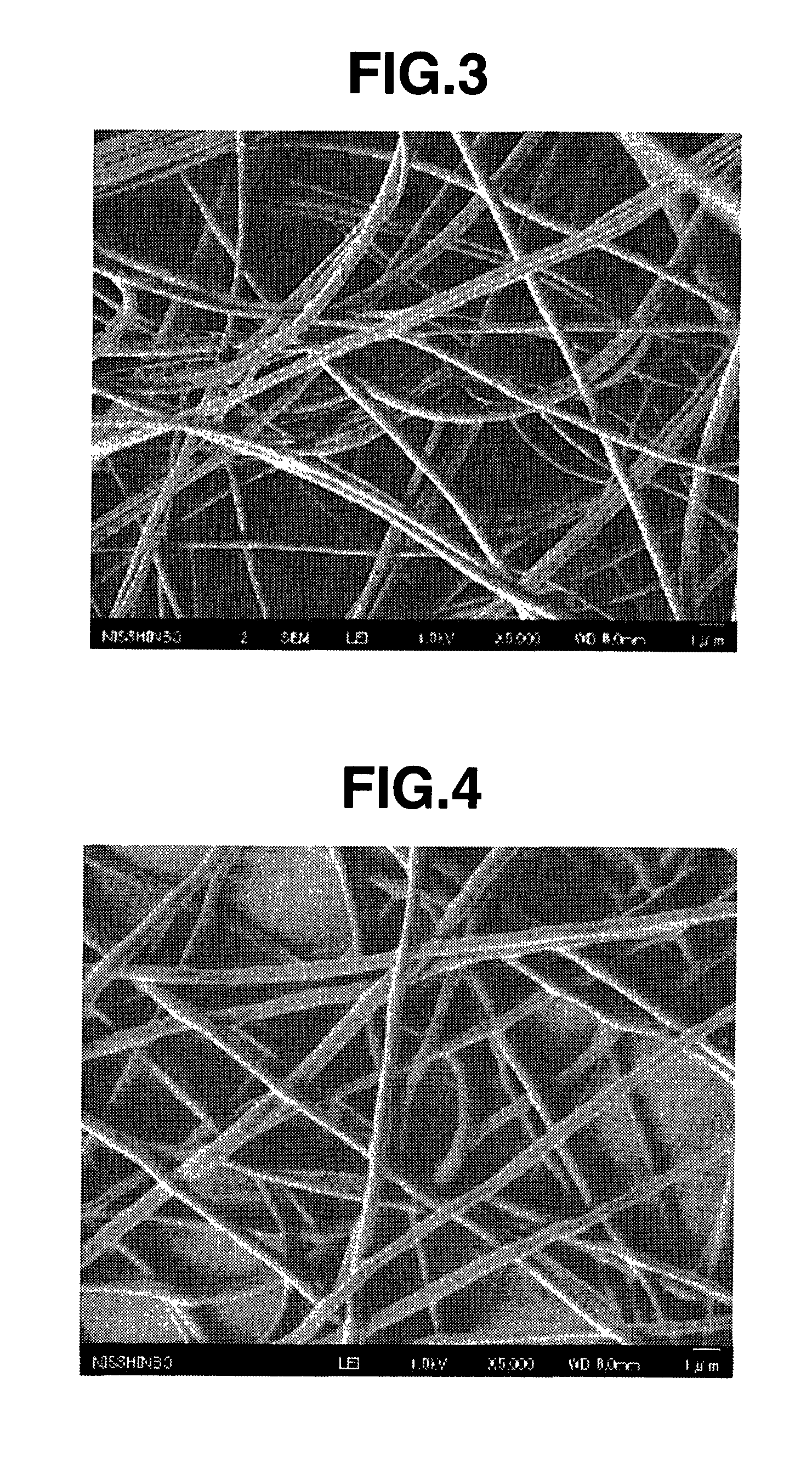
[0134]The resulting nonwoven fabric had an average fiber diameter of 500 nm, and fibers with a diameter greater than 3 μm were not observed. The nonwoven fabric had a thickness of 100 μm and a basis weight of 15 g / m2. FIG. ...
example 2
Nylon 66
[0142]Ten parts by weight of nylon 66 (Amilan (registered trademark) CM3001-N; manufactured by Toray Industries, Inc.) was dissolved in 57 parts by weight of formic acid at room temperature (25° C.), thereby obtaining 67 parts by weight of a nylon 66-containing solution (solids content, 15 wt %).
[0143]This nylon 66-containing solution (spinning dope) was placed in a syringe and electrostatic spinning was carried out at a discharge tip orifice diameter of 0.4 mm and an applied voltage of 50 KV (at room temperature and atmospheric pressure), thereby giving a nanofiber nonwoven fabric. The resulting nonwoven fabric had an average fiber diameter of 250 nm, and fibers with a diameter greater than 1 μm were not observed. The nonwoven fabric had a thickness of 50 μm and a basis weight of 2.0 g / m2.
[0144]The ratio A2 / A1 of the peak height A2 near 1640 cm−1 to the peak height A1 near 1550 cm−1 in an infrared absorption spectrum of this nonwoven fabric was 1.7.
Bond Energy Ratio
[0145]Fr...
example 3
Nylon 6
[0149]Aside from using nylon 6 (A1030BRT, produced by Unitika, Ltd.) instead of nylon 66, a nanofiber nonwoven fabric was obtained in the same way as in Example 2.
[0150]The resulting nonwoven fabric had an average fiber diameter of 300 nm, and fibers with a diameter greater than 1 μm were not observed. The nonwoven fabric had a thickness of 50 μm and a basis weight of 3.5 g / m2.
[0151]The ratio A2 / A1 of the peak height A2 near 1640 cm−1 to the peak height A1 near 1550 cm−1 in an infrared absorption spectrum of this nonwoven fabric was 1.7.
Bond Energy Ratio
[0152]From Table 1 above, at 25° C., the C—H bond energy is 416 kJ / mol, the C—C bond energy is 357 kJ / mol, the C═O bond energy is 804 kJ / mol, the C—N bond energy is 273 kJ / mol, and the N—H bond energy is 391 kJ / mol.
[0153]The total bond energy is thus 416×10+357×5+804+273+391=7,413 kJ / mol.
[0154]The electron-withdrawing group bond energy is 804+273+391=1,468 kJ / mol.
[0155]Therefore, the bond energy ratio is 1,468 / 7,413=0.20.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com