Method of producing fluoroapatite, fluoroapatite, and adsortion apparatus
a technology of adsorption apparatus and fluoroapatite, which is applied in the direction of phosphorus halides/oxyhalides, chemistry apparatus and processes, and other chemical processes, can solve the problems of difficult to remove ammonia from particles, difficult to obtain particles having uniform characteristics, and ammonia remains, so as to improve the acid resistance of the produced fluoroapatite and crystallize high. the effect of high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
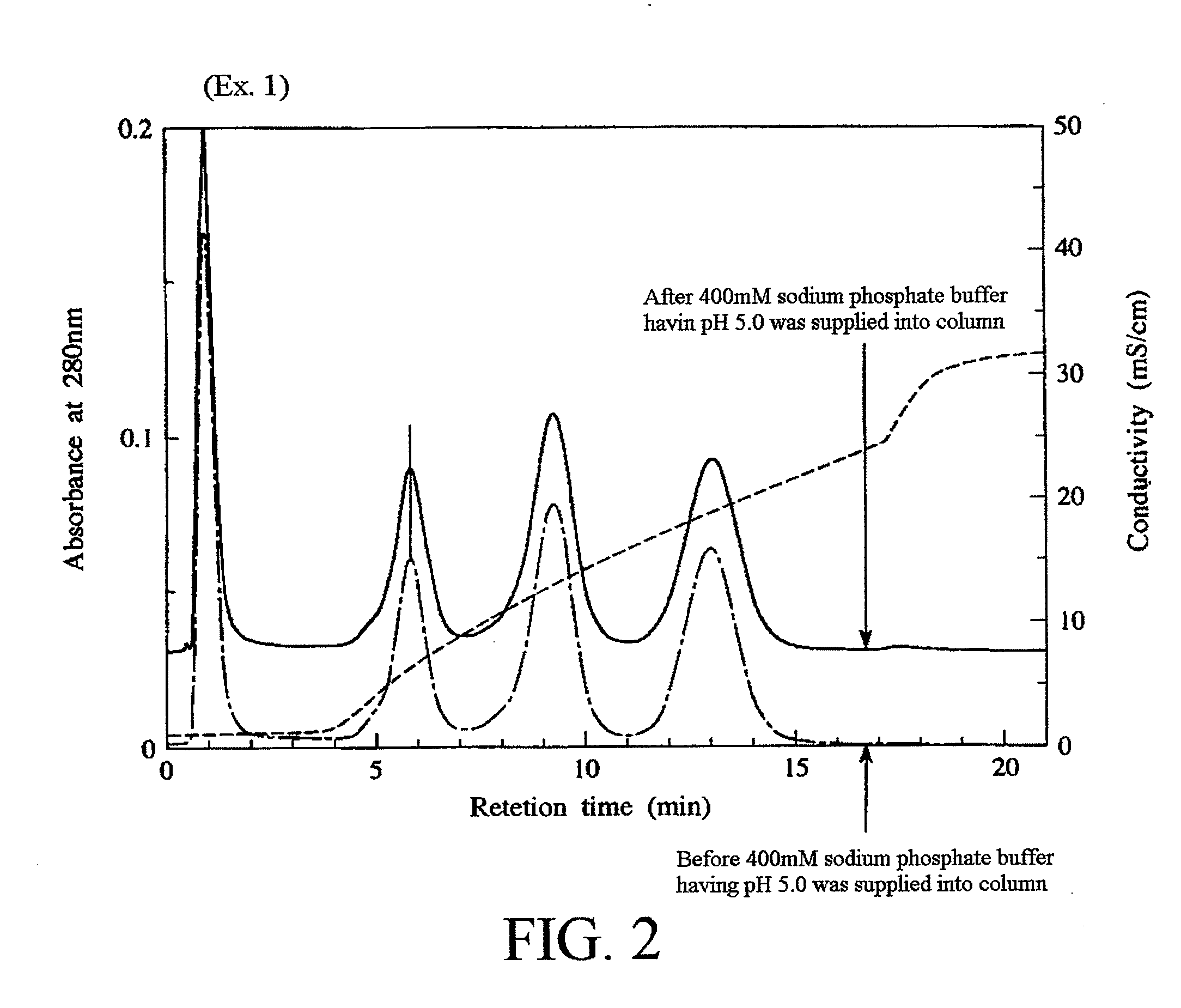
example 1
[0099]First, calcium hydroxide was suspended in pure water to obtain a calcium hydroxide suspension, and then an aqueous phosphoric acid solution was dropped into the calcium hydroxide suspension while the calcium hydroxide suspension was sufficiently stirred. As a result, 500 L of a slurry containing 10 wt % of hydroxyapatite primary particles was obtained.
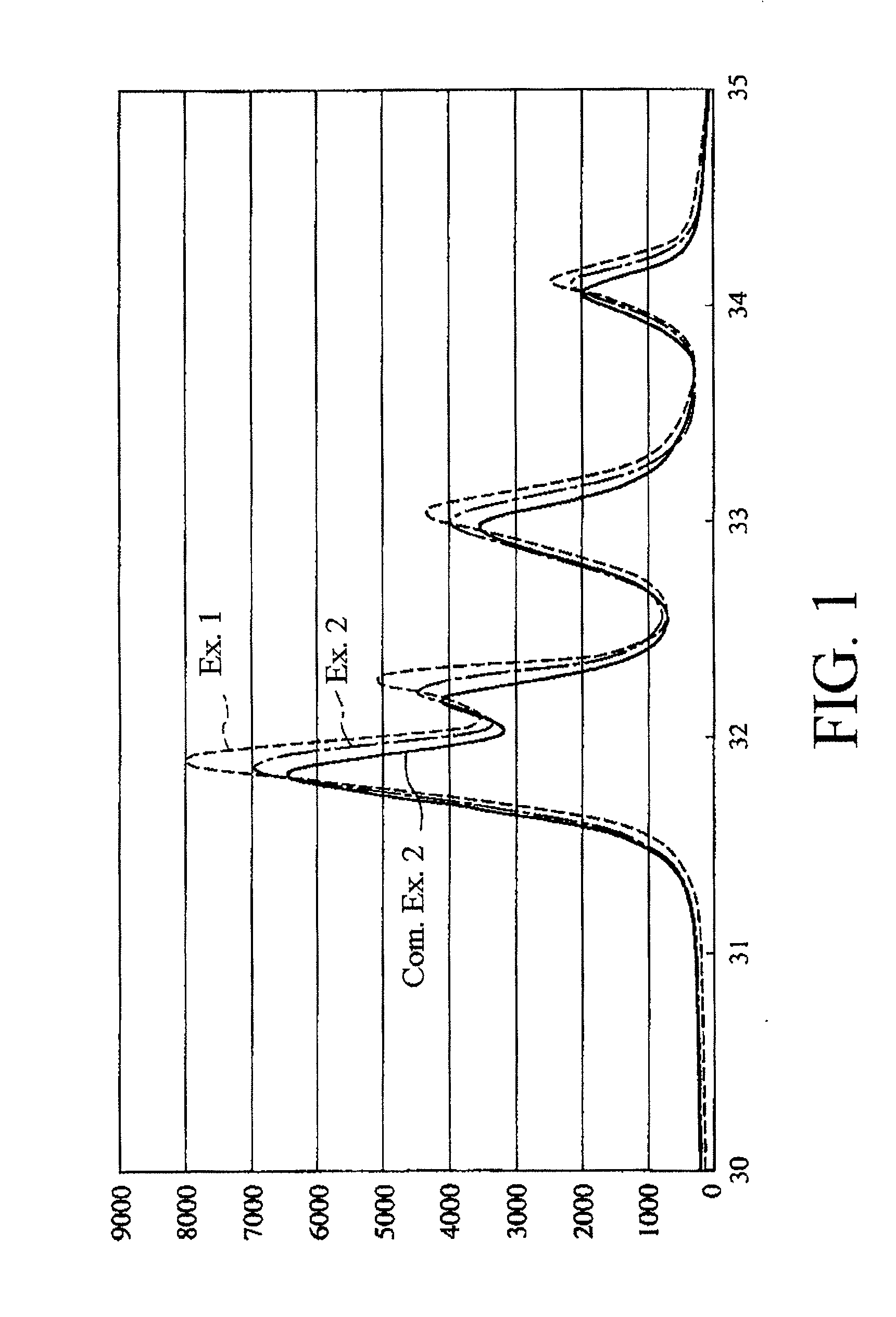
[0100]It is to be noted that the thus obtained hydroxyapatite primary particles were found to be hydroxyapatite by powder X-ray diffractometry.
[0101]On the other hand, hydrogen fluoride was dissolved in pure water so that an amount thereof is 5 wt % to prepare a hydrogen fluoride-containing solution.
[0102]Then, 41.84 L of the hydrogen fluoride-containing solution was dropped into the slurry at a rate of 5 L / hr while the slurry was stirred at a stirring power of 1 kW.
[0103]It is to be noted that the slurry had a pH of 3.00 at the time when the dropping of the hydrogen fluoride-containing solution was completed. An amount of the hy...
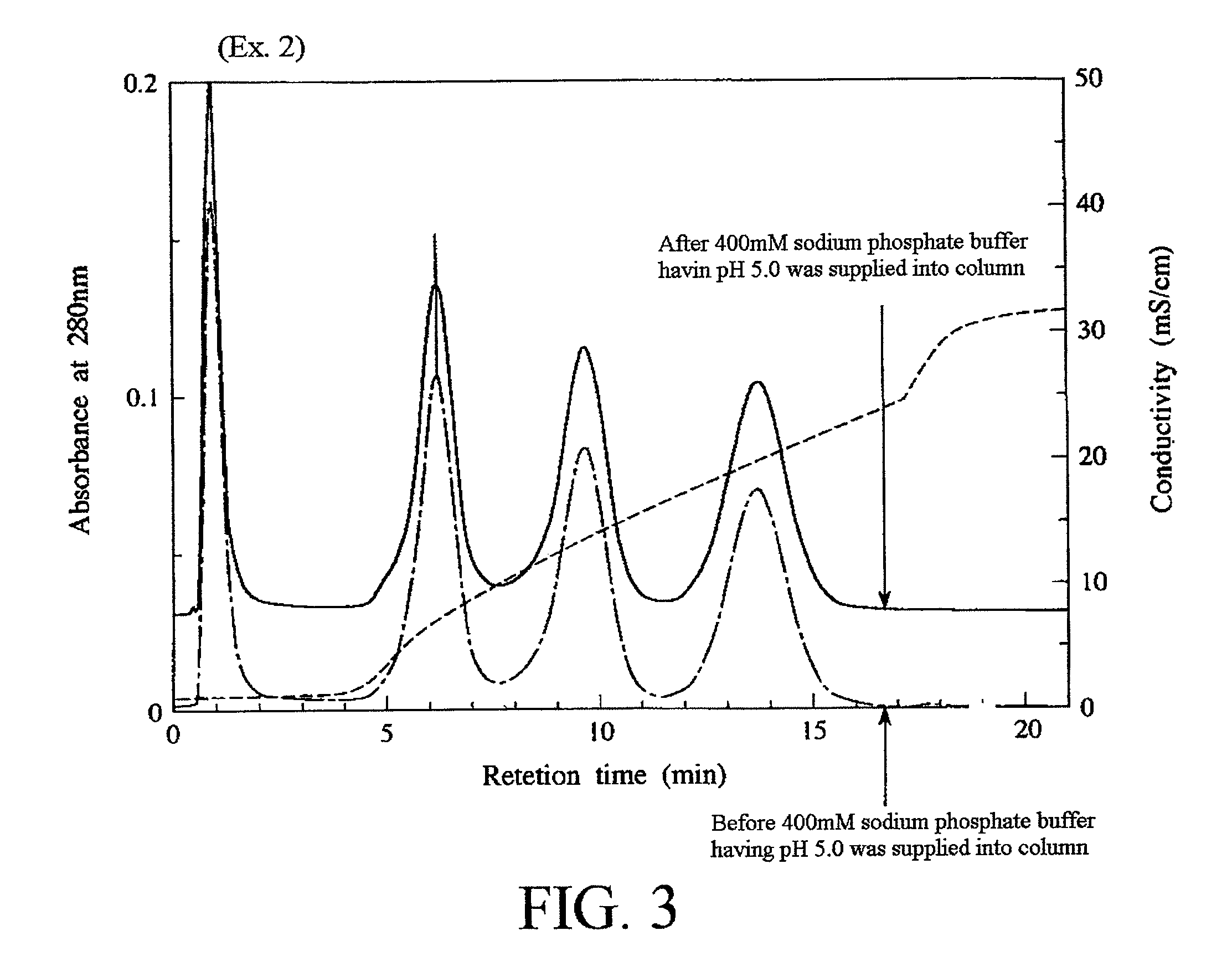
example 2
[0110]Fluoroapatite primary particles, fluoroapatite dried particles, and fluoroapatite sintered particles 1 and 2 were produced in the same manner as in the Example 1 except that the pH of the slurry at the time of completion of the dropping of the hydrogen fluoride-containing solution was adjusted to 3.36 by adding pure water to the hydrogen fluoride-containing solution.
[0111]It is to be noted that the fluoroapatite primary particles had a rate of substitution of hydroxyl groups by fluorine atoms of about 75%. Further, as a result of powder X-ray diffraction of the fluoroapatite dried particles, any products other than the fluoroapatite were not detected.
[0112]It is also to be noted that the fluoroapatite dried particles had an average particle size of about 40 μm, and each of the two kinds of the fluoroapatite sintered particles 1 and 2 (adsorbents) also had an average particle size of about 40 μm.
example 3
[0113]Fluoroapatite primary particles, fluoroapatite dried particles, and fluoroapatite sintered particles 1 and 2 were produced in the same manner as in the Example 1 except that the pH of the slurry at the time of completion of the dropping of the hydrogen fluoride-containing solution was adjusted to 3.96 by adding pure water to the hydrogen fluoride-containing solution.
[0114]It is to be noted that the fluoroapatite primary particles had a rate of substitution of hydroxyl groups by fluorine atoms of about 50%. Further, as a result of powder x-ray diffraction of the fluoroapatite dried particles, any products other than the fluoroapatite and the hydroxyapatite were not detected.
[0115]It is also to be noted that the fluoroapatite dried particles had an average particle size of about 40 μm, and each of the two kinds of the fluoroapatite sintered particles 1 and 2 (adsorbents) also had an average particle size of about 40 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com