Radical scavenger and active oxygen eliminating agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Lipid Peroxide-Suppressing Action
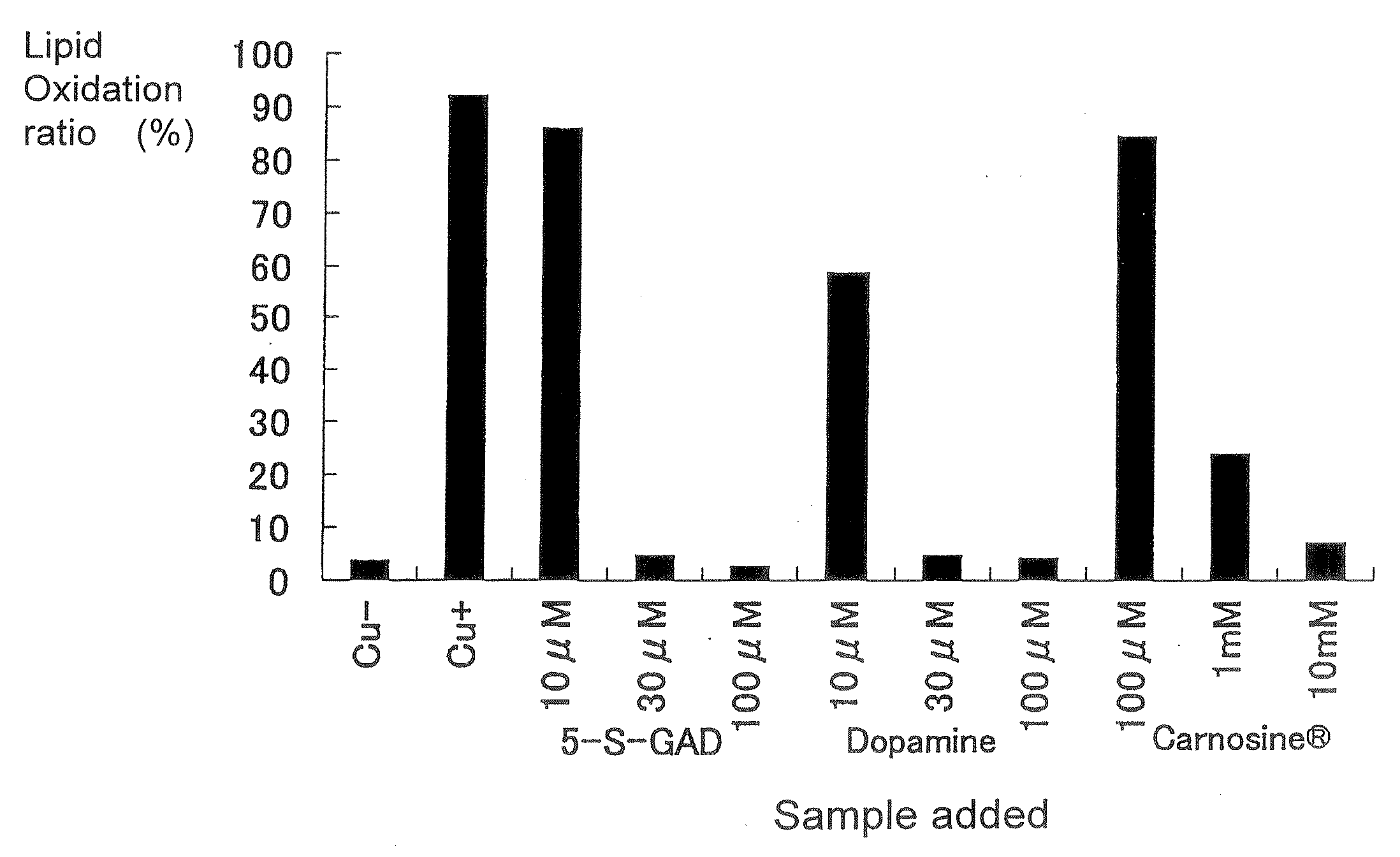
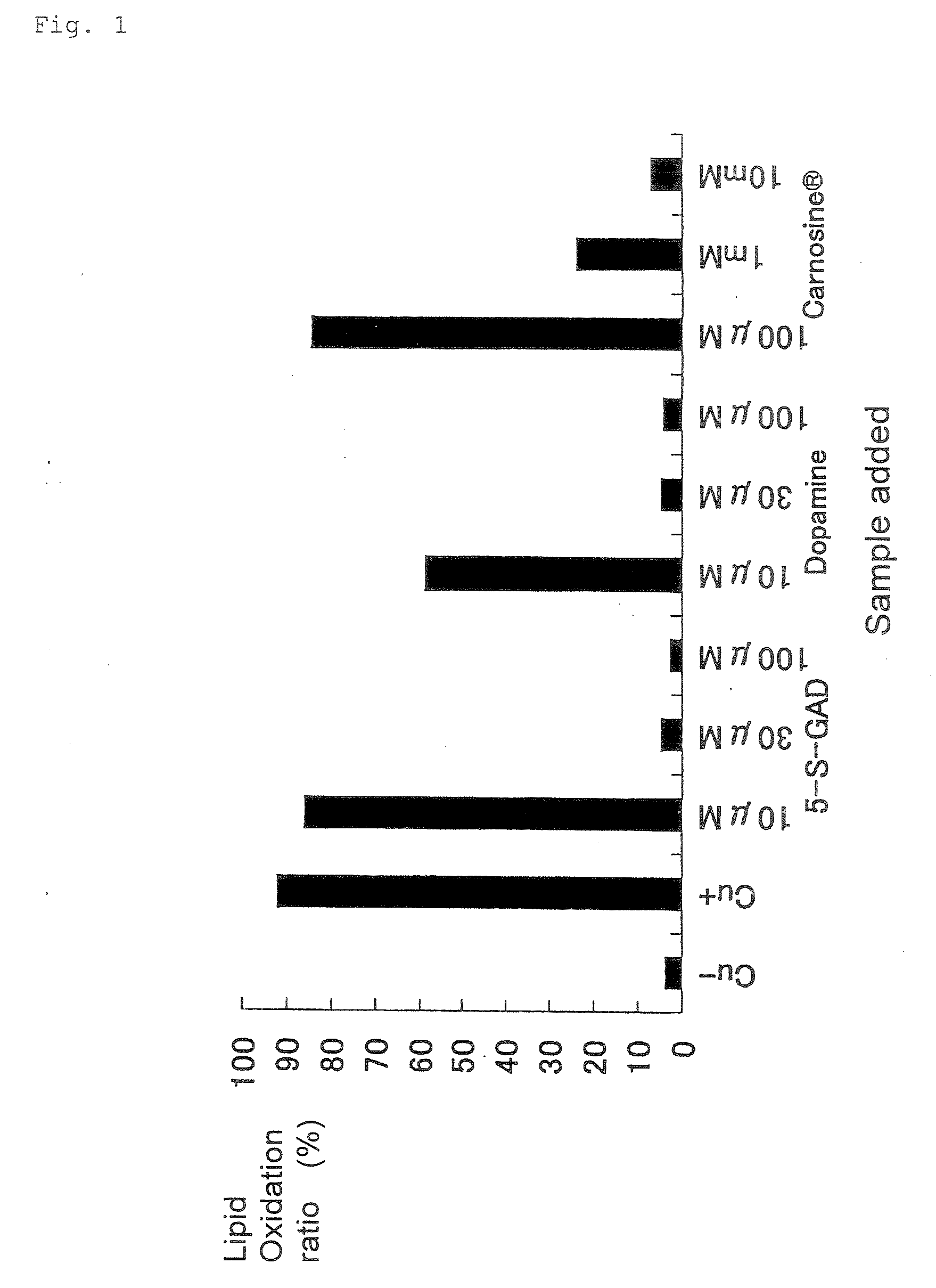
[0062]As an action of capturing free group (free radical-scavenging action), the effect of N-β-alanyl-5-S-glutathionyl-3,4-dihydroxyphenylalanine (5-S-GAD) on LDL oxidation was examined. As controls, dopamine and Carnosine (under trade name; N-acetylcarnosine as the ingredient name) used as eye drops for cataract were used. LDL was prepared by a fractionation and centrifugation method. A fraction at a specific gravity of 1.019 to 1.063 g / mL in serum was defined as human LDL. 5 μM CuSO4, 5-S-GAD (3 to 100 μM) and N-acetyl-carnosine (100 μM to 10 mM) or L-carnosine (10 μM to 10 mM) were added to 0.2 mg / mL LDL, for incubation at 37° C. for 3 hours. After termination of the incubation, TBARS (a substance reactive with thiobarbituric acid) was assayed. Although the TBA reaction is a non-specific reaction, the reaction is used for a method of assaying various lipid peroxide products including malone dialdehyde. An experiment of peroxidation with copper sul...
example 2
DPPH-Scavenging Action
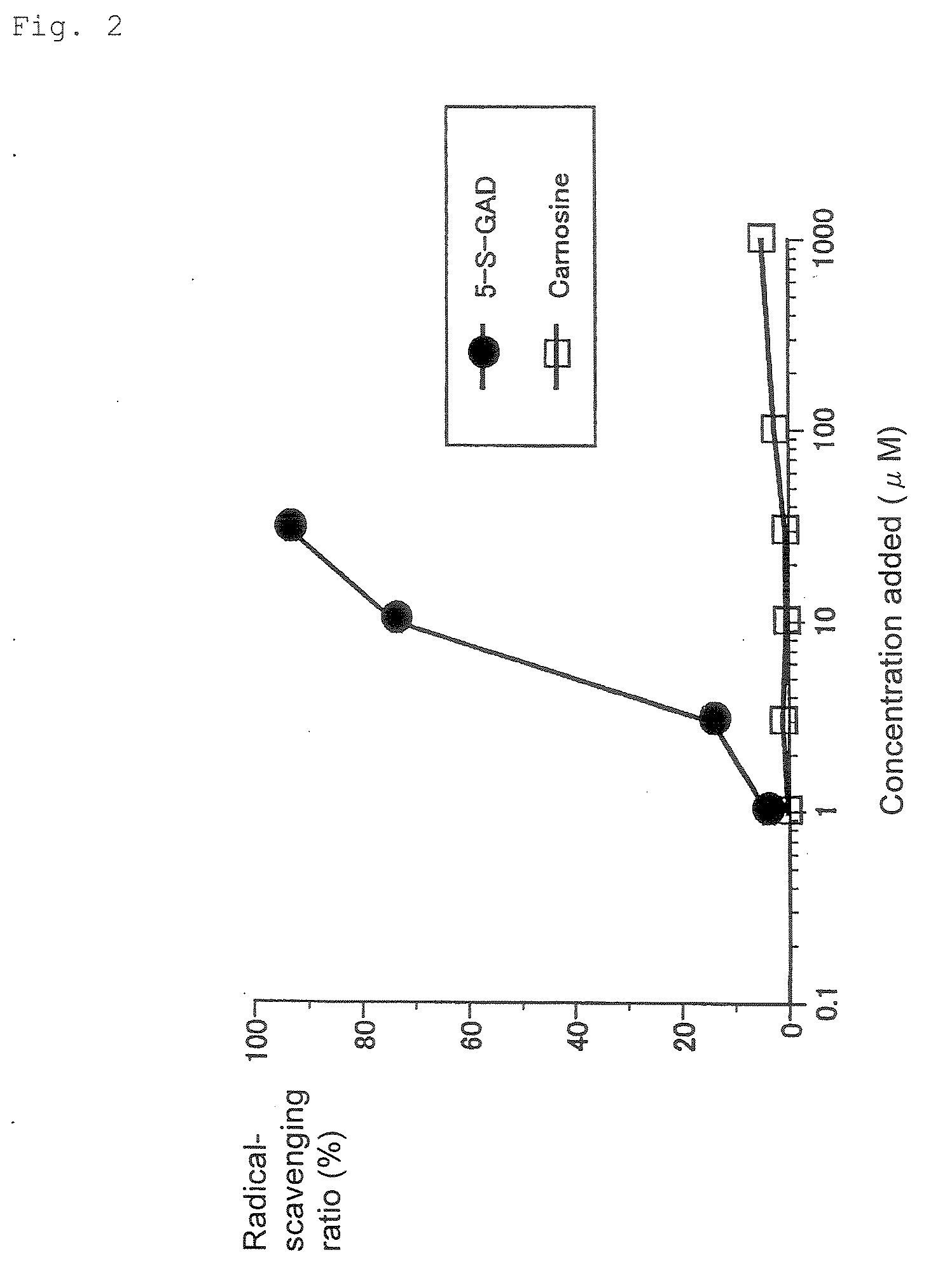
[0064]DPPH (1,1-diphenyl-2-picrylhydrazyl) is one of nitrogen radicals and is a very stable radical, which is commercially available as a black-purple crystal (Wako Pure Chemical, Co., Ltd., etc.). N-β-Alanyl-5-S-glutathionyl-3,4-dihydroxyphenylalanine (5-S-GAD) (see Table 2) or Carnosine (under trade name) (see Table 3) was added to an ethanol solution of 50 mM DPPH radical, for reaction at ambient temperature for 5 minutes (see the following reaction scheme). Instead of the test reagent, distilled water was added to the ethanol solution, which was used as a background. The DPPH absorbance at 517 nm was assayed, to calculate the scavenging ratio (%) of the DPPH radical according to the following equation. The scavenging ratio was defined as radical scavenger activity (mean, n=2; see Table 2, Table 3 and FIG. 2).
Radical-scavenging ratio (%)=[assay value of background (mean)−assay value of solution at each concentration] / background assay value (mean)]×100
TABLE 2...
example 3
Superoxide Anion (O2−)-Scavenging Activity
[0066]In a 50 mM carbonate buffer (pH 10) containing 200 μM lucigenin, 100 μM xanthine and 1 mU / mL xanthine oxidase reacted together. The chemiluminescence level depending on the generated superoxide anion (O2−) was assayed with BIOLUMAT (under trade name) (Model type LB 9505, manufactured by EG&G BERTHORD Company) for 10 minutes. The area under the curve indicating the total chemiluminescence over the 10 minutes was calculated. Defining the area under the curve for a group (control) added with PBS(−) instead of the test substance solution at the same amount as that of the solution as 100, the effect of N-β-alanyl-5-S-glutathionyl-3,4-dihydroxyphenylalanine (5-S-GAD) (Table 4) or Carnosine (under trade name) (Table 5) on the increase of chemiluminescence was examined. The suppressive activity thereof was defined as superoxide anion-scavenging activity (%) (mean, n=2; see Table 4, Table 5 and FIG. 3).
TABLE 41) 5-S-GADConcentration added (μM)R...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com